Introduction
Outsourced bioanalytical services play a critical role in helping early-stage biotech companies overcome scientific, operational, and regulatory challenges. In the early phases of drug development, startups often face limited budgets, small internal teams, and intense pressure to generate high-quality data quickly. Partnering with a specialized bioanalytical CRO enables these companies to access advanced analytical capabilities without the burden of building in-house infrastructure.
ResolveMass Laboratories Inc. delivers end-to-end bioanalytical services in drug development, supporting discovery, preclinical, and clinical programs. This article explains why early-stage biotech companies outsource bioanalytical services to CROs, the advantages of this model, and how ResolveMass supports reliable, regulator-ready bioanalysis.
Summary
Early-stage biotech companies increasingly rely on outsourced bioanalytical services to accelerate drug development while controlling costs and regulatory risk. The main reasons include:
- Faster access to specialized scientific expertise
- Reduced capital expenditure on instruments and infrastructure
- Regulatory-ready data generation for IND-enabling studies
- Scalability to support evolving pipelines
- Improved data quality, compliance, and reproducibility
- Focus on core R&D and strategic innovation
Outsourcing bioanalysis through partners offering affordable bioanalytical services for biotech startups allows emerging biotech organizations to advance confidently from discovery to clinical development.
1: What Are Outsourced Bioanalytical Services?
Outsourced bioanalytical services refer to contracting specialized laboratories to perform quantitative and qualitative analysis of drugs, metabolites, biomarkers, and biologics in biological matrices.
These services typically include:
- PK/TK and bioavailability studies
- Bioanalytical method development and bioanalytical method validation
- PK/PD bioanalysis and toxicokinetic bioanalysis
- Biomarker bioanalytical services and large molecule bioanalysis
- Stability, matrix evaluation, and sample integrity studies
- Regulatory-compliant reporting through regulated bioanalytical services
- Stability and sample integrity studies
All services are delivered through specialized bioanalytical laboratory services.
For early-stage biotech companies, outsourcing bioanalytical services ensures access to validated methods and experienced scientists without maintaining internal labs.
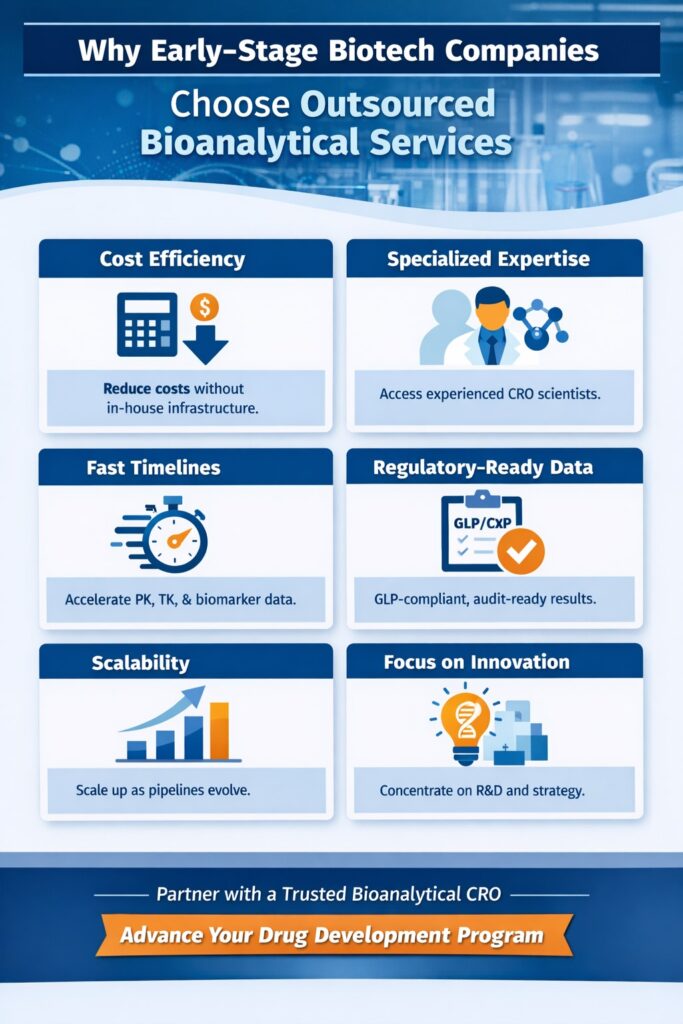
2: Why Early-Stage Biotech Companies Choose Outsourced Bioanalytical Services
2.1 Cost Efficiency Without Compromising Quality
Early-stage biotech companies outsource bioanalytical services to avoid the high cost of maintaining in-house analytical infrastructure. Instrumentation, staffing, and compliance significantly increase overall bioanalytical testing services cost.
Cost savings include:
- No capital expenditure on LC-MS/MS platforms
- No long-term staffing commitments
- Reduced maintenance and validation costs
- Predictable project-based pricing
This makes bioanalytical outsourcing a financially sound strategy.
Outsourced bioanalytical services provide enterprise-level capabilities at a fraction of the cost.
2.2 Immediate Access to Specialized Expertise
Outsourcing bioanalytical services gives biotech startups immediate access to highly experienced scientists.
CRO teams bring hands-on experience from multiple therapeutic areas, molecule types, and regulatory pathways.
Expertise typically covers:
- Complex biological matrices
- Small molecule vs large molecule bioanalysis
- Small and large molecule quantification
- Regulatory expectations for IND and NDA submissions
- LC-MS/MS bioanalysis of xenobiotics
- Challenging sensitivity requirements
- LC-MS for large molecules
- Biosimilar bioanalysis
- Cell and gene therapy bioanalysis
ResolveMass Laboratories Inc. applies deep scientific knowledge to solve real-world analytical challenges efficiently.
2.3 Faster Timelines and Accelerated Development
Speed is a key reason early-stage biotech companies outsource bioanalytical services. CROs operate validated workflows supported by high-throughput bioanalysis, enabling faster turnaround of PK, TK, and biomarker data.
Benefits include:
- Rapid method development and qualification
- Parallel processing of multiple studies
- Faster turnaround for PK and toxicology data
This acceleration can be critical when meeting investor milestones or regulatory timelines.
2.4 Regulatory-Ready Data from Day One
Outsourced bioanalytical services ensure alignment with global regulatory expectations. CROs provide compliant workflows and documentation supporting bioanalytical services for IND and NDA submissions.
Professional CROs provide:
- GLP and GxP-aligned workflows
- FDA, EMA, and ICH-compliant validation
- Complete audit-ready documentation
ResolveMass also supports the transition between discovery vs regulated bioanalysis, reducing regulatory risk.
2.5 Scalability as Pipelines Evolve
Outsourcing bioanalytical services offers flexibility as biotech pipelines expand or pivot.
Early-stage companies often experience rapid changes in project scope and study design.
Outsourced models allow:
- Easy scale-up from discovery to preclinical
- Bioanalytical services outsourcing for pharma
- Support for multiple programs simultaneously
- Adaptation to new molecular modalities
- Clinical bioanalytical services
- Bioanalytical services in North America
This scalability helps biotech companies stay agile without overextending internal resources.
2.6 Focus on Core Innovation and Strategy
By outsourcing bioanalytical services, biotech founders can focus on innovation instead of operations.
Running an analytical lab requires ongoing attention to quality systems, audits, and maintenance.
Outsourcing allows internal teams to concentrate on:
- Target discovery and biology
- Lead optimization and formulation
- Strategic partnerships and fundraising
This strategic focus is essential for early-stage success.
3: Common Bioanalytical Services Outsourced by Early-Stage Biotech Companies
| Bioanalytical Area | Purpose | Typical Stage |
|---|---|---|
| LC-MS/MS Quantification | Drug concentration analysis | Discovery–Preclinical |
| PK/TK Studies | Exposure and dose optimization | Preclinical |
| Biomarker Analysis | Proof of mechanism | Preclinical–Early Clinical |
| Method Validation | Regulatory readiness | IND-enabling |
| Stability Studies | Sample integrity | Preclinical–Clinical |
These services highlight why bioanalysis is important for data-driven drug development.
4: How Outsourced Bioanalytical Services Support E-E-A-T
Outsourcing mitigates analytical risk caused by challenges in bioanalytical method development and issues such as bioanalytical matrix effects.
- Experience: Real-world biotech program exposure
- Expertise: Advanced bioanalytical quantification
- Authoritativeness: Documented regulated workflows
- Trustworthiness: Reproducible, audit-ready data
4.1 Demonstrated Experience
CROs like ResolveMass Laboratories Inc. bring real-world experience from diverse biotech programs, strengthening the reliability of generated data.
4.2 Proven Expertise
Highly trained analytical scientists ensure accurate method development, validation, and interpretation.
4.3 Authoritative Processes
Established SOPs, quality systems, and regulatory alignment reinforce authority in bioanalysis.
4.4 Trustworthy Results
Transparent communication, reproducible data, and audit-ready documentation build long-term trust with biotech partners.
5: Choosing the Right CRO for Outsourced Bioanalytical Services
Not all CROs are equal. Early-stage biotech companies should evaluate partners based on:
- Regulatory compliance history
- Data integrity and reproducibility
- Communication and project transparency
- Breadth of bioanalytical services
- Experience as a dedicated CRO
- Regulatory compliance history
- Flexibility for early-stage programs
ResolveMass provides a comprehensive bioanalytical services overview aligned with early-stage needs.
6: Why ResolveMass Laboratories Inc. Is a Trusted Partner
ResolveMass Laboratories Inc. provides outsourced bioanalytical services tailored to the needs of early-stage biotech companies. With advanced LC-MS/MS capabilities, experienced scientists, and a strong commitment to data quality, ResolveMass supports confident decision-making at every development stage.
Key strengths include:
- Customized bioanalytical strategies
- Regulatory-aligned validation approaches
- High-sensitivity and complex assay expertise
- Clear communication and collaborative workflows
Conclusion
Outsourced bioanalytical services are no longer optional for early-stage biotech companies—they are essential. By partnering with experienced CROs, biotech startups gain access to expertise, speed, compliance, and scalability without sacrificing quality or control. Outsourcing bioanalysis allows emerging companies to focus on innovation while ensuring their data meets the highest scientific and regulatory standards.
ResolveMass Laboratories Inc. stands as a trusted partner for biotech organizations seeking reliable, regulatory-ready outsourced bioanalytical services that support long-term success.
Frequently Asked Questions:
Pharma companies use CROs to access specialized scientific expertise, reduce development costs, and accelerate drug development timelines. CROs provide validated processes, regulatory-compliant data, and scalable support across discovery, preclinical, and clinical stages. By outsourcing to CROs, pharma companies can focus on core innovation while minimizing operational and regulatory risk.
Outsourced bioanalytical services involve hiring specialized CRO laboratories to analyze drugs, metabolites, biomarkers, and biologics in biological matrices using validated analytical methods. This approach allows biotech companies to access advanced expertise and compliant data generation without maintaining in-house laboratories.
CRO outsourcing is the practice of contracting a Contract Research Organization to perform specific research, development, or analytical activities on behalf of a pharmaceutical or biotech company. It allows organizations to leverage external expertise, advanced infrastructure, and compliant workflows without maintaining in-house capabilities. CRO outsourcing improves efficiency, flexibility, and data quality across the drug development lifecycle.
Early-stage biotech companies outsource bioanalytical services to reduce costs, accelerate timelines, and ensure regulatory-ready data. Outsourcing provides immediate access to specialized expertise, validated methods, and scalable support across discovery, preclinical, and clinical stages.
Yes. Professional CROs provide bioanalytical services for IND and NDA submissions that meet FDA, EMA, and ICH expectations. This includes validated methods, regulated workflows, and audit-ready documentation to support successful regulatory filings.
Outsourcing bioanalytical services allows biotech companies to scale analytical support as pipelines grow without expanding internal infrastructure. CROs can support multiple programs simultaneously and adapt quickly to new study designs or therapeutic modalities.
Reference
- Xiaojing Yu &Arkady I Gusev.CRO Benchmarking for Clinical Biomarker Analysis Outsourcing.https://www.tandfonline.com/doi/full/10.4155/bio-2019-0123
- Scott G Summerfield,Christopher Evans,Neil Spooner,John A Dunn,Matthew E Szapacs &Eric Yang.Integrating Internal and External Bioanalytical Support to Deliver A Diversified Pharmaceutical Portfolio.https://www.tandfonline.com/doi/abs/10.4155/bio.14.93
- Aimin TanORCID Icon,Constantine Fanaras &John C Fanaras.Efficient interactions with bioanalytical contract research organizations: inside scoop.https://www.tandfonline.com/doi/full/10.4155/bio-2022-0129
- Graeme T Clark.The Importance of Partnership When Outsourcing Exploratory Bioanalysis.https://www.tandfonline.com/doi/full/10.4155/bio.14.10

