Part I: Poly Glycidol Fundamentals and Molecular Architecture Control
Section 1: Poly Glycidol (PG) in Polymer Therapeutics
1.1 Chemical Structure and Key Physico-Chemical Properties of PG
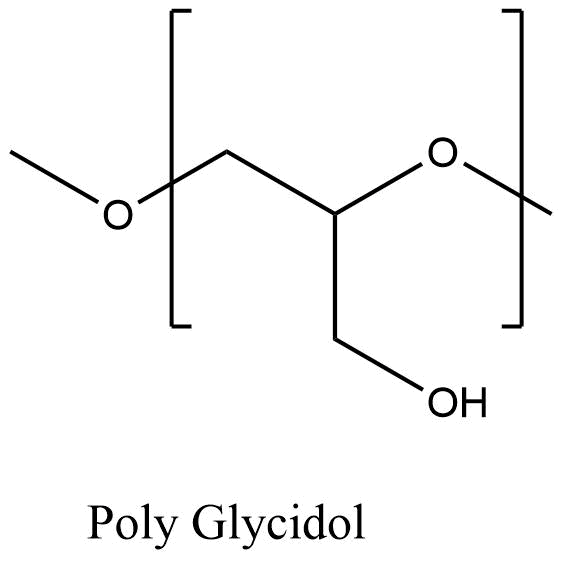
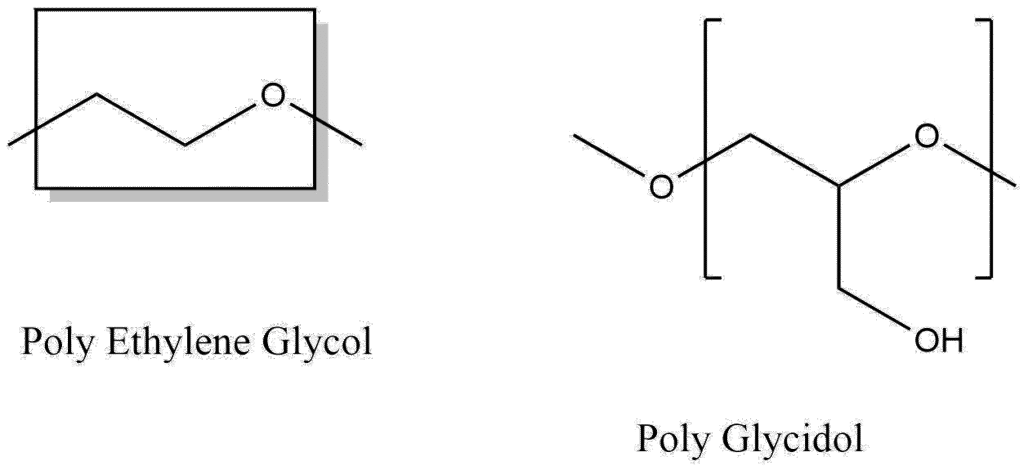
Poly Glycidol (PG), also known as polyglycerol, represents a high-potential class of polymer therapeutics structurally akin to poly(ethylene oxide) (PEG), the industry standard. The defining characteristic that differentiates PG from PEG is the presence of a pendant −CH2OH reactive side group attached to every structural unit of the main polymer chain. This dense arrangement of hydroxyl groups is critical to the polymer’s performance in biomedical applications.
The inherent presence of multiple hydroxyl functionalities along the flexible polyether backbone confers several advantages. First, these groups significantly enhance the polymer’s water-solubility, making PG highly hydrophilic. Second, the high functional density provides numerous sites for post-polymerization modification, allowing for extensive customization, conjugation chemistries (e.g., drug loading, attachment of targeting ligands), and the construction of complex polymer architectures. The resultant polymers exhibit excellent biocompatibility, a prerequisite for advanced drug delivery systems.
1.2 PG vs. PEG: Addressing Immunogenicity and Polydispersity Challenges
For decades, PEG has dominated drug delivery systems due to its hydrophilicity and established biocompatibility, improving the stability and pharmacokinetics (PK) of numerous drugs. However, persistent limitations associated with PEG have spurred the exploration of alternatives like PG. Conventional PEG is fundamentally a statistical mixture of polymers, resulting in inherent molecular heterogeneity and high polydispersity. This heterogeneity complicates purification and drug synthesis and is one factor contributing to emerging concerns regarding hypersensitivity, nonbiodegradability, and the production of anti-PEG antibodies (immunogenicity).
The necessity for PG as a successor hinges entirely on the consistency and quality achievable through controlled synthesis. While research has focused on generating uniform (monodisperse) PEGs to overcome these limitations, the procedures are costly, iterative, and purification-intensive. PG offers a structural alternative that intrinsically addresses some of these biological issues. Specifically, certain PG architectures, such as hyperbranched polyglycidol (HPG), have demonstrated superior immunological profiles. Studies involving HPG-grafted nanoparticles revealed that they induced minimal anti-polymer immunoglobulin M (IgM) responses and exhibited no accelerated blood clearance (ABC) effects in test subjects, a notable advantage over PEG, particularly for repeat-dose therapies. Furthermore, PGL derivatives have been shown to exhibit significantly enhanced hydrophilic properties compared to conventional PEG, which translates biologically to reduced cellular uptake by macrophages when coated onto nanoparticles. This reduced cellular interaction contributes directly to the observed stealth properties, confirming that PG’s unique structure provides a clear performance benefit in critical areas where PEG is increasingly challenged.
1.3 Versatile Architectures: Linear, Hyperbranched, and Star PG Rationale
Polyglycidol can be synthesized into various architectural forms, each tailored for specific functional requirements in polymer therapeutics. The two most common forms are linear and hyperbranched:
- Hyperbranched Polyglycidol (HPG): This architecture results from the direct polymerization of the glycidol monomer and is the natural outcome due to the monomer’s dual reactivity. HPG structures are highly dense polyols characterized by a large number of primary and secondary hydroxyl groups on their surface. This structure is favored for applications requiring high functional loading and bulk modification, acting as a scaffold or dendritic core.
- Linear Polyglycidol (LPG): Achieving a linear structure is chemically demanding. LPG requires polymerizing a glycidol monomer where the pendant hydroxyl group has been selectively blocked. Linear PG is often preferred for precise, monodisperse drug conjugation, mimicking the desired linearity of advanced PEG conjugates but potentially avoiding the immunogenicity pitfalls.
- Star-like PG: More complex architectures can be accessed by employing multifunctional initiators during the polymerization of blocked glycidol. The resultant star polymer’s arm number is precisely controlled by the number of hydroxyl groups present in the initiating alcohol.
Section 2: Controlled Polymerization Techniques and Synthesis Routes
2.1 Glycidol Reactivity and the Challenge of Branching
The primary synthetic challenge of PG arises from the bifunctionality of the glycidol monomer, which contains both an epoxide group and a hydroxyl group. In anionic polymerization, which is a standard method for controlling polymer architecture, the epoxide ring is the desired site for chain propagation. However, the hydroxyl group simultaneously acts as a potent participant in chain transfer reactions, both intermolecularly (between polymer chains) and intramolecularly (within a single chain). This inherent chain transfer mechanism is the root cause of the formation of branched structures. Consequently, hyperbranched PG (HPG) is the typical product unless extraordinary measures are taken to control or eliminate this side reaction.
2.2 Anionic Ring-Opening Polymerization (AROP) using Protected Monomers (Route to Linear PG)
To overcome the branching challenge and synthesize linear PG (LPG) with controlled molecular weight and narrow polydispersity index (PDI), chemists must employ a strategy utilizing a protected glycidol monomer. The pendant hydroxyl group must be blocked using protecting groups such as 1-ethoxyethyl or trityl moieties prior to the AROP reaction.
In this controlled approach, highly efficient initiators are necessary. For the polymerization of blocked glycidol, systems such as cesium hydroxide or mono- and difunctional potassium alkoxides, often coupled with powerful sec-BuLi/phosphazene base combinations, have been successfully used. The formation of the linear polymer structure is achieved only after the controlled AROP is complete, followed by a final, highly selective deprotection step, usually carried out under acidic conditions to remove the blocking group.
For more insights on how specialized polymerization techniques are tailored for client-specific targets, explore our detailed overview of Custom Synthesis Services and our Comprehensive Guide to Custom Synthesis Service, which outlines how synthetic route design and reagent protection strategies are optimized for complex polymer architectures.
2.3 Cationic Polymerization: Mechanisms and Catalyst Selection
Glycidol polymerization can also be achieved through cationic means. This process typically proceeds via the active chain-end (ACE) mechanism or the activated monomer (AM) mechanism. The ACE mechanism tends to yield polymers that retain exclusively primary side hydroxyl groups. Conversely, in systems initiated by Brønsted acids, the AM mechanism is significant; the activated glycidol can react readily with hydroxyl groups already along the polymer chain, leading to inevitable branching and the formation of both primary and secondary hydroxyl groups in the resulting polymer.
Furthermore, specialized catalysts like tris-(pentafluorophenyl)borane initiate a zwitterionic ring expansion polymerization. This mechanism produces branched PG, often featuring a cyclic core structure. A complicating factor in custom synthesis using B(C6F5)3 is the frequent occurrence of heterogeneous polymerization. In poor solvents for PG (such as toluene, which is otherwise a good solvent for the glycidol monomer), the polymer precipitates rapidly during the reaction, carrying the catalyst into the solid phase. This shift from a homogenous reaction mixture to heterogeneous kinetics dramatically complicates process control and reproducibility on a large scale. The industrial challenge lies in characterizing and controlling the continuation of the reaction in the precipitated phase, where molecular weight growth occurs through chain fusion events, ensuring the final batch consistently meets the client’s specifications for mass distribution, despite the deviation from ideal homogeneous kinetics.
2.4 The Role of Superbases and Living Polymerization for Narrow PDI Control
For applications such as bioconjugation, consistency in the polymer’s molar mass distribution is non-negotiable, often requiring a narrow PDI (typically <1.1) to ensure reliable drug loading, predictable stability, and consistent pharmacokinetic performance. Achieving this control necessitates implementing living polymerization techniques.
Nitrogen–phosphorous hybrid organobases, commonly known as phosphazene bases (PBs), have emerged as critical tools in AROP for this purpose. These non-ionic superbases are remarkably powerful, generating highly reactive anionic species and accelerating polymerization rates significantly compared to methods relying solely on metal cations. The use of protonated or non-protonated phosphazenium counterions facilitates polymerization control that typically results in polymers exhibiting narrow molecular weight distributions and well-defined end groups. This level of control is essential for custom synthesis projects aiming to produce pharmacologically active polymers that meet stringent clinical standards.
2.5 Chemoselective Functionalization and Protecting Group Strategies
Polyglycidol, especially linear PG (LPG) where a high proportion of primary hydroxyls are available, often requires selective functionalization to attach complex payloads or targeting moieties. This process demands precise control over the reactivity of the hydroxyl groups. Achieving this differential reactivity requires the implementation of chemoselective protection and deprotection strategies.
A robust custom synthesis strategy leverages various protecting groups chosen for their differential stability against different chemical environments. Common groups include trityl, triethylsilyl (TES), and tert-butyldimethylsilyl (TBDMS) ethers. For instance, TES ethers can be selectively deprotected using mild reagents, such as formic acid in methanol, leaving TBDMS ethers completely unaffected. Conversely, TBDMS ethers are stable to aqueous base but are readily removed under controlled acidic conditions. This careful selection and differential removal of protective groups allow for the sequential modification of different reactive sites, a capability that is paramount for constructing complex, high-value PG conjugates used in advanced polymer therapeutics.
Table I summarizes the key comparative features essential for custom synthesis planning when choosing a PG architecture.
Table I: Comparative Properties of Poly Glycidol (PG) Architectures and PEG (Detailed for Custom Synthesis Planning)
| Property | Linear Poly Glycidol (LPG) | Hyperbranched Poly Glycidol (HPG) | Conventional Poly(ethylene glycol) (PEG) |
| Structure | Linear polyether backbone with pendant primary hydroxyls | Highly branched, dense polyol structure | Linear polyether chain |
| Synthesis Route | AROP of Protected Glycidol (e.g., EEGE) | Direct AROP/CROP of Glycidol | Polymerization of Ethylene Oxide |
| PDI Control Potential | Excellent (PDI < 1.1 achievable, via living AROP with superbases) | Moderate (PDI typically > 1.3, due to chain transfer) | Variable (Conventional PDI > 1.05) |
| Functionalization Sites | Terminal and pendant primary hydroxyls | High density of surface hydroxyls | Terminal hydroxyls only |
| Immunogenicity Risk | Minimal/Low | Minimal/Very Low (ABC effect avoidance observed) | Emerging concerns (Anti-PEG antibody formation) |
| Complexity of Synthesis | High (Multi-step: Protection, Polymerization, Deprotection) | Moderate (Single-step polymerization) | Low to Moderate |
Part II: Custom Synthesis Process, Route Optimization, and Scale-Up
Section 3: Strategic Synthetic Route Selection for Custom PG
3.1 Custom Synthesis Project Lifecycle and Phasing
A custom synthesis project follows a defined project management lifecycle comprising several key phases :
- Initiation: This initial stage defines the project’s scope, cost, feasibility, goals, timeline, and success criteria. For PG, this phase is critical for determining the viability of achieving a specific architecture (LPG versus HPG), the tightest acceptable PDI limits, and the complexity of end-group functionalization.
- Planning: A detailed action plan and synthetic roadmap are created. This includes the crucial initial consultation and the intricate design of the most efficient synthetic route, often incorporating novel chemistry developed specifically for the client.
- Execution: The laboratory-scale synthesis is carried out, followed by rigorous analytical validation.
Custom synthesis contracts are often structured around specific financial models based on the project’s inherent risk and complexity. The Fee-for-Service (FFS) model is standard for projects with well-defined scopes and predictable outcomes, often for short-term proof-of-concept synthesis. Conversely, the Full-Time Equivalent (FTE) model is highly advantageous for complex R&D. The FTE approach allows a dedicated team of chemists to explore more speculative and innovative chemistry solutions, which is essential for projects requiring sophisticated architectural control, such as low PDI LPG. This model charges a set monthly fee, providing flexibility to add or remove chemistry targets as project priorities evolve.
The planning and execution phases of polymer synthesis projects often mirror broader custom chemistry workflows. Researchers new to this process may find value in our resource, What is Custom Synthesis? A Comprehensive Guide for Researchers, which explains how scope definition, budget alignment, and IP protection form the foundation of successful project delivery.
3.2 Application of the SELECT Criteria for Process Chemistry Optimization
The selection of the synthetic route carries the largest impact on the economic and environmental performance of the final manufacturing process. All proposed PG synthesis routes must be rigorously evaluated against the industry-standard SELECT criteria:
- S – Safety: Given that the AROP of glycidol derivatives often employs highly reactive initiators (such as superbases, CsOH, or organometallics) , the route must prioritize minimizing reactive hazards, toxicity, and the use of hazardous reagents and solvents.
- E – Environmental: The chosen route should minimize environmental impact by reducing the volume and hazardous nature of waste and solvents.
- L – Legal: Comprehensive Intellectual Property (IP) due diligence is required. IP ownership clauses within the custom contract must clearly define proprietary rights, patent rights, trade secret protections, and confidentiality obligations related to any novel chemistry developed.
- E – Economics: The route must be optimized to minimize the cost of goods (CoG) while meeting quality targets.
- C – Control: The manufacturing process must be validated, consistently meet quality specifications, and produce a consistent impurity profile.
- T – Throughput: Consideration must be given to maximizing the space-time yield, manufacturing time efficiency, and ensuring the stable availability of raw materials.
The decision regarding the financial model must align with chemical complexity. Highly innovative PG synthesis, such as the multi-step AROP of protected glycidol using superbases to achieve low PDI LPG, inherently involves elevated technical risk. Since the FTE model explicitly supports “speculative and innovative chemistry solutions” by dedicating resources to problem-solving , it is the optimal choice for the Route Design and Optimization phase. Relying on an FFS model for such ill-defined, complex R&D carries a high risk of scope creep and financial disagreements if unexpected chemical hurdles arise.
Route optimization under the SELECT framework aligns closely with the decision-making principles discussed in Custom Synthesis CRO Services, where operational safety, scalability, and regulatory compliance determine the viability of industrial synthesis models.
3.3 Process Intensification and Safety: Transitioning to Continuous Flow
Traditional polymer and small molecule synthesis often relies on conventional batch reactors. However, the process development phase must assess advanced technologies for improved efficiency and safety. Continuous flow systems offer significant advantages over batch processes for PG synthesis, primarily through improved heat and mass transfer. These improvements positively influence conversion rates, reproducibility, and overall throughput.
The greatest benefit of flow chemistry is realized when scaling processes involving hazardous or highly exothermic reactions, such as the AROP of glycidol or the use of reactive reagents. Continuous flow architecture allows for the reduction of the total volume of reactive material present at any time, significantly mitigating the risk of catastrophic failure (e.g., thermal runaway) associated with large batch volumes of hazardous chemistry. Custom synthesis providers leverage flow chemistry to manage hazardous chemistries like nitration, ozonation, or those involving reactive gases and potent initiators, ensuring inherently safer operation.
Continuous flow chemistry represents one of the most transformative advances in polymer manufacturing. To see how digitalization and automation are accelerating innovation in this area, refer to our article on How AI is Revolutionizing Custom Polymer Synthesis.
Section 4: Process Scale-Up, Engineering, and Safety Assessment
4.1 Critical Differences Between Laboratory and Pilot/Commercial Scale
Scale-up is an iterative process requiring a fundamental understanding of how reaction variables change with volume. The inherent risk is substantial, with scale-up incidents frequently involving thermal runaway, explosions, and fires. Simply multiplying reagent quantities fails to account for critical physical and chemical phenomena that manifest only at larger volumes.
Pilot programs, utilizing specialized equipment ranging from 50 to 3000 gallons , are essential for bridging the gap between bench chemistry and commercial manufacturing. These programs serve to test industrial-scale technologies and identify scale-dependent phenomena, including changes in heat transfer efficiency, localized mixing limitations, fouling rates, catalyst aging, corrosion issues, and the need for specific environmental controls. Critical scale-up variables requiring re-evaluation include the absolute quantity of reactants, reaction time, and thermal management efficiency.
4.2 Mandatory Process Safety Screening: Thermal Hazard and Runaway Risk Analysis
Due to the exothermic nature of many polymerization reactions, a complete thermal hazard assessment is mandatory before scaling any PG process. Uncontrolled exothermic events can lead to dangerous accumulation of heat or pressure. Custom synthesis providers must employ a dedicated Process Safety Lab equipped with advanced calorimetry tools:
- Reaction Calorimetry (RC1): This technique simulates process conditions at laboratory scale, measuring heat flow and enabling the classification of the reaction’s criticality.
- Accelerating Rate Calorimeter (ARC): The ARC is vital for thermal screening, determining the kinetics of decomposition, and pinpointing the onset temperature of thermal runaway.
- Vent Sizing Package (VSP2): For processes that generate significant gas volume or pressure (common in polymerization), the VSP2 generates data crucial for calculating and specifying emergency pressure relief systems (vent sizing) in accordance with DIERS methodology.
The data generated by these assessments informs the design of necessary safety controls for the manufacturing plant, such as emergency cooling mechanisms, quenching procedures, or controlled depressurization protocols. This proactive approach ensures safety is built into the process design from the ground up, minimizing risk prior to final scale-up.
Many of the principles applied during PG scale-up—such as calorimetric safety testing and thermal runaway prediction—also extend to modern controlled polymerization methods. To understand how different polymerization routes are chosen and optimized, see our discussion on RAFT vs ATRP: Choosing the Best Method for Custom Polymer Synthesis.
4.3 Hazard Analysis and Operability (HAZOP) and Technology Transfer
Prior to full execution, all scaled processes undergo a rigorous Hazard Analysis and Operability (HAZOP) review to systematically ensure operational safety.
Successful movement of a synthetic route from development to manufacturing requires a meticulous Technology Transfer Package (TTP). Knowledge transfer is the central component of this process. The TTP must include a detailed Process Description from the sending site, meticulously outlining all process parameters, conditions, and Standard Operating Procedures (SOPs). Crucially, the TTP must also account for equipment differences, recognizing that the receiving facility will likely not possess identical equipment models. Careful planning and comprehensive documentation of critical quality attributes (CQAs) at every step are paramount for managing the inherent risks in technology transfer.
Section 5: Quality Assurance and Regulatory Documentation
5.1 Adherence to cGMP Standards for Intermediates and Final Polymer
For any PG intended for pharmaceutical or clinical application, adherence to current Good Manufacturing Practice (cGMP) guidelines is mandatory. cGMP governs both production and quality control, guaranteeing consistency and reproducibility. The complexity of PG synthesis involves multiple intermediates (e.g., protected glycidol, PG oligomers) that must be precisely managed. Well-characterized intermediates are frequently required as analytical reference materials for impurity profiling, method development, and validation during formal regulatory submissions.
When synthesizing highly potent PG derivatives (High-Potency Active Pharmaceutical Ingredients, HPAPIs), the manufacturing environment requires specialized high-containment suites, optimized scale-up protocols, and rigorously validated cleaning and change-over procedures to prevent operator exposure and cross-contamination.
5.2 Essential Documentation: Batch Records and Certificates of Analysis (CoA)
The foundation of quality assurance is documentation. The final step of any custom synthesis project requires the generation of meticulous records, including detailed batch records and the Certificate of Analysis (CoA).
Batch Record Review (BRR) is a fundamental component of cGMP compliance. Batch records provide a complete history of the manufacturing process, verifying that every step, including materials used, processing steps, and deviation handling, was executed according to approved Standard Operating Procedures (SOPs). This documentation serves as both a legal document and the proof required by Quality Assurance (QA) prior to batch release. Expert custom synthesis providers offer advanced documentation support that goes beyond a standard CoA and spectra, providing full synthesis protocols, comprehensive impurity profiling, and supporting method validation data to aid the client’s regulatory filing.
Containers holding PG intermediates or the final API must be labeled clearly with the name, batch number, critical storage conditions, and the expiry or retest date to ensure quality throughout the supply chain.
5.3 Intellectual Property (IP) Considerations in Custom Manufacturing Agreements
Intellectual Property (IP) ownership must be precisely defined within the custom synthesis contract, especially where novel PG synthetic pathways or intermediates are developed. IP ownership clauses are essential for establishing proprietary rights, specifying patent rights, trade secret protections, and confidentiality obligations related to the specific process. Reputable synthesis providers not only deliver the physical product but also provide expert documentation and consultation, which can be critical for supporting IP claims related to API synthesis and impurity profile replication in subsequent legal or regulatory contexts.
Part III: Advanced Analytical Profiling and Impurity Control
6.1.1 Structural Elucidation by NMR and Elemental Analysis
Definitive characterization of PG architecture, especially following complex functionalization or deprotection steps, demands orthogonal analytical techniques. Structural confirmation requires a combination of Elemental Analysis (EA), HRMS, and Nuclear Magnetic Resonance (NMR) spectroscopy. NMR capabilities, including 1D and 2D experiments (1H, 13C, 19F, 31P-NMR, HSQC, HMBC, COSY, NOESY), are essential for confirming linkage integrity, verifying the ratio of primary versus secondary hydroxyl groups, and validating the successful introduction of specific end-group functionalizations.
Conclusions
The custom synthesis of Poly Glycidol (PG) requires an integrated mastery of advanced polymer chemistry, robust chemical engineering principles, and highly sensitive analytical techniques to successfully position PG as a superior alternative to Poly(ethylene glycol) (PEG) in polymer therapeutics.
- Architectural Control is Paramount: PG’s potential lies in its ability to mitigate the immunogenicity issues associated with heterogeneous PEG. Achieving this requires rigorous synthetic control, mandating the use of protected glycidol monomers and superbase-initiated living polymerization to reliably produce Linear PG with a narrow PDI, a complex multi-step process that often aligns best with the FTE financial model during R&D.
- Process Safety is the Scale-Up Gatekeeper: The exothermic nature of polymerization necessitates mandatory process safety screening using tools like Reaction Calorimetry (RC1) and Vent Sizing Package (VSP2). The data generated from these thermal hazard assessments dictates the engineering design for cooling and venting, thereby governing the feasibility and safety of commercial scale-up. The potential benefits of continuous flow chemistry for mitigating these thermal risks must be explored during route optimization.
To learn more about how advanced polymer synthesis workflows—like those used for controlled Poly Glycidol architectures—are executed from concept to GMP-compliant production, visit our series on Custom Synthesis and Polymer CRO Services and Comprehensive Guide to Custom Synthesis Service
References:
- Gosecki M, Gadzinowski M, Gosecka M, Basinska T, Slomkowski S. Polyglycidol, Its Derivatives, and Polyglycidol-Containing Copolymers-Synthesis and Medical Applications. Polymers (Basel). 2016 Jun 9;8(6):227. doi: 10.3390/polym8060227. PMID: 30979324; PMCID: PMC6432134.
2. Pouyan, P., Cherri, M., & Haag, R. (2022). Polyglycerols as Multi-Functional Platforms: Synthesis and Biomedical Applications. Polymers, 14(13), 2684. https://doi.org/10.3390/polym14132684
3. Dworak, A., Slomkowski, S., Basinska, T., Gosecka, M., Walach, W., & Trzebicka, B. (2013). Polyglycidol—how is it synthesized and what is it used for?. Polimery, 58(9), 641–649. Retrieved from https://polimery.ichp.vot.pl/index.php/p/article/view/771
4. Hyperbranched Polyglycerols: From the Controlled Synthesis of Biocompatible Polyether Polyols to Multipurpose ApplicationsDaniel Wilms, Salah-Eddine Stiriba, and Holger FreyAccounts of Chemical Research 2010 43 (1), 129-141 DOI: 10.1021/ar900158p