OVERVIEW of 1,4-Butane-d8-diamine | CAS 709-608-92-4
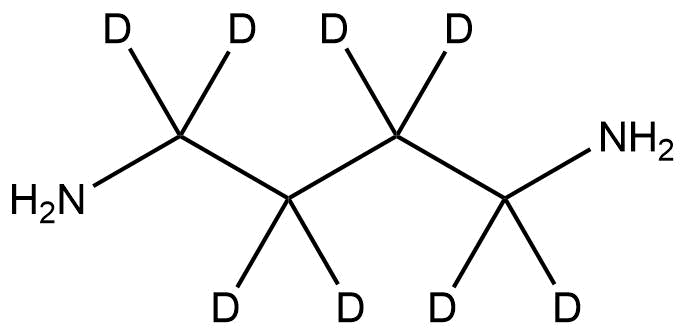
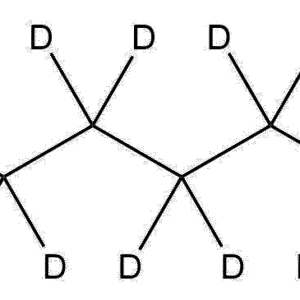
1,4-Butane-d₈-diamine (CAS 709608-92-4) is a fully deuterated analog of 1,4-butanediamine (putrescine), in which all eight hydrogen atoms along the carbon backbone are replaced with deuterium. This compound is primarily used as a stable isotope-labeled reagent for advanced research applications in analytical chemistry, biochemistry, and metabolomics. Its deuterated structure allows precise differentiation from the non-deuterated counterpart in mass spectrometry and NMR spectroscopy studies.
CHEMICAL INFORMATION
-
Name: 1,4-Butane-d₈-diamine
-
Molecular Formula: C₄D₁₂N₂
-
Molecular Weight: 96.20 g/mol
-
CAS Number: 709608-92-4
-
Isotopic Enrichment: ≥ 98 atom % D
-
Chemical Class: Deuterated aliphatic diamine
-
Solubility: Miscible with water, ethanol, and other polar solvents
APPLICATIONS of 1,4-Butane-d8-diamine | CAS 709-608-92-4
-
Mass Spectrometry (MS):
Commonly used as an internal standard for quantifying native putrescine and related diamines. The isotopic shift from deuterium enables clear signal separation for accurate quantitation. -
NMR Spectroscopy:
The absence of proton signals from the carbon chain reduces spectral interference, allowing detailed chemical shift analysis and reaction monitoring. -
Metabolic and Biochemical Research:
Utilized as a stable isotope tracer to study polyamine biosynthesis, metabolism, and uptake pathways in cellular and microbial systems. -
Synthetic Chemistry:
Acts as a deuterated intermediate for the preparation of labeled polymers, surfactants, or biologically active molecules, aiding isotopic labeling strategies in pharmaceutical and materials science. -
Pharmacokinetic Studies:
Employed to trace the distribution and transformation of diamines and polyamines in biological matrices with high analytical precision.
ADVANTAGES of 1,4-Butane-d8-diamine | CAS 709-608-92-4
-
Fully deuterated (8 deuterium atoms) for clear isotopic distinction.
-
Non-radioactive and chemically stable.
-
Excellent reproducibility for quantitative isotope-dilution studies.
-
Compatible with aqueous and organic analytical systems.
-
Useful for mechanism elucidation and molecular tracing experiments.
HANDLING AND SAFETY
-
Handling: Use in a well-ventilated environment; avoid inhalation and direct contact.
-
Protective Equipment: Wear gloves, protective eyewear, and lab coat.
-
Storage: Keep in sealed containers under inert atmosphere; protect from air and moisture.
-
Stability: Stable under recommended conditions; may absorb moisture upon exposure.
-
Disposal: Dispose of according to local environmental and chemical safety regulations.
SUMMARY
1,4-Butane-d₈-diamine (CAS 709608-92-4) is a high-purity, fully deuterated diamine designed for use as an isotopic standard, NMR reference, and tracer compound in chemical and biological research. Its excellent stability, high deuterium enrichment, and close structural match to native putrescine make it indispensable for quantitative mass spectrometry, metabolic tracing, and mechanistic studies.
Learn more about,
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Availability of All the Deuterated Chemicals at ResolveMass Laboratories Inc.
ResolveMass Laboratories: Leading Deuterated Chemical Synthesis Company in the United States.
Deuterated Internal Standards for LC-MS: Selection & Custom Synthesis
How to Choose the Right Deuterated Labelled Chemical Synthesis Company in Canada
How to Choose the Right Deuterium Labelled Compounds Supplier for Your Lab
Deuterium-Labelled Compounds — Synthesis, Applications & Ordering
Custom Synthesis of Deuterated Chemicals
Reviews
There are no reviews yet.