OVERVIEW
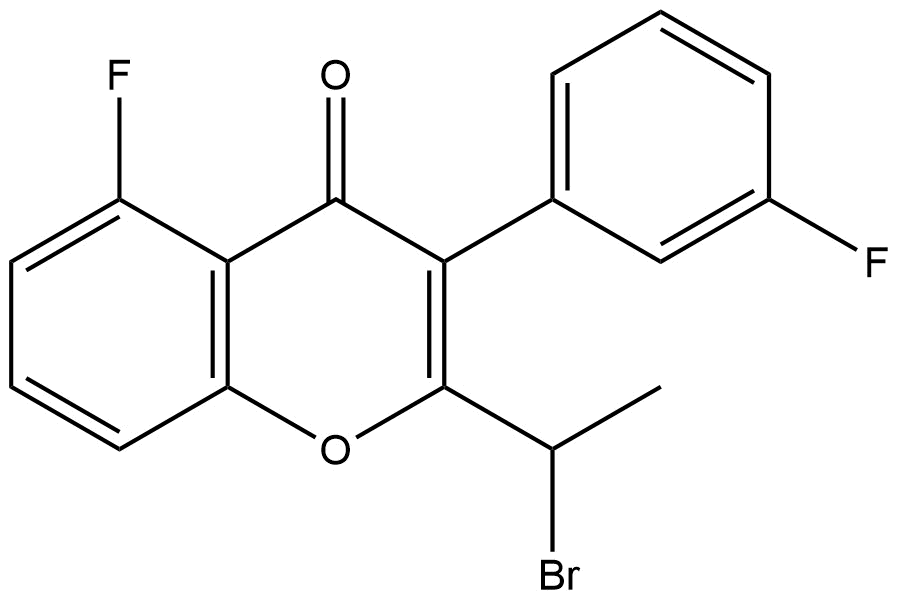
Chemical Name: 2‑(1‑bromoethyl)‑5‑fluoro‑3‑(3‑fluorophenyl)‑4H‑chromen‑4‑one
CAS Number: 1408087‑79‑5
Molecular Formula: C17H11BrF2O2
Molecular Weight: 365.17 g/mol (approx.)
Synonyms:
-
5‑Fluoro‑3‑(3‑fluorophenyl)‑2‑(1‑bromoethyl)‑4H‑chromen‑4‑one
-
Bromoethylfluorochromenone derivative
-
Fluorophenyl bromochromenone
2-(1-bromoethyl)-5-fluoro-3-(3-fluorophenyl)-4H-chromen-4-one | CAS 1408087-79-5 is an advanced fluorinated chromenone derivative featuring multiple aromatic moieties and a bromine substituent. Its unique structural attributes make it valuable as an intermediate in medicinal chemistry, fluorine‑enabled property modulation studies, and synthetic methodology research.
CHEMICAL STRUCTURE & FEATURES
Structural Highlights:
-
Chromenone Core: A bicyclic scaffold combining a benzene ring with an α‑pyrone (chromen‑4‑one) system.
-
Fluoro Substitution: Two fluorine atoms — one on the chromenone ring (5‑position) and one on the phenyl ring (meta position) — which can influence electronic properties, metabolic stability, and lipophilicity.
-
Bromoethyl Side Chain: A 1‑bromoethyl substituent at the 2‑position serves as a reactive handle for further functionalization through nucleophilic substitution and cross‑coupling chemistries.
This combination yields a molecule with distinctive reactivity and physicochemical properties that attract interest for advanced chemical research.
PHYSICAL & CHEMICAL PROPERTIES (TYPICAL/ESTIMATED)
| Property | Value (Typical/Estimated) |
|---|---|
| Appearance | Off‑white to pale solid |
| Molecular Weight | 365.17 g/mol |
| Solubility | Soluble in organic solvents (e.g., DMSO, DMF, acetone); limited aqueous solubility |
| Chemical Stability | Stable under recommended storage; avoid moisture and strong nucleophiles |
| Reactivity | Electrophilic site at bromine; amenable to substitution reactions |
Note: Exact melting point and spectral data (NMR, MS, IR) are available upon request or in supplied documentation.
SYNTHETIC APPLICATIONS & RESEARCH USE
This compound is primarily intended as a research intermediate in synthetic and medicinal chemistry. Its design supports a range of research objectives:
Medicinal Chemistry & Lead Optimization
-
Fluorine Effects: Fluorine incorporation is a well‑established strategy to improve metabolic stability and alter biological recognition in drug candidates. Fluoroaryl and fluoroheterocyclic scaffolds are prevalent in modern therapeutic agents.
-
Chromenone Derivatives: Chromen‑4‑one moieties are common in bioactive compounds with anticancer, antifungal, anti‑inflammatory, and antioxidant profiles.
-
Bromine Handle for Diversification: The reactive bromine substituent enables further derivatization via Suzuki, Heck, or nucleophilic substitution reactions to generate libraries of analogues for structure‑activity relationship (SAR) studies.
Method Development & Synthetic Methodologies
-
Cross‑Coupling Studies: The bromoethyl group is valuable for testing or developing nickel, palladium, or copper catalysis for C–C and C–heteroatom bond formation.
-
Fluorination Techniques: The presence of multiple fluorinated positions offers opportunities for exploring regioselective transformations and fluorination methodologies.
Agrochemical and Material Science Explorations
While primarily a pharmaceutical intermediate, the chromenone framework and electron‑modulating substituents may support early exploratory research in:
-
Agrochemical lead compounds
-
Fluorine‑rich materials with unique optical or electronic features
POTENTIAL DERIVATIZATION ROUTES
The 2‑(1‑bromoethyl) substituent enables numerous transformations:
Nucleophilic Substitution
-
Reaction with amines to form ethanamines
-
Reaction with thiols to generate thioethers
Cross‑Coupling Reactions
-
Suzuki–Miyaura coupling with boronic acids/esters
-
Heck reactions for C=C bond formation
-
Sonogashira coupling with alkynes
These pathways make the compound a flexible precursor for analogue synthesis.
DOCUMENTATION & QUALITY ASSURANCE
ResolveMass Laboratories Inc. provides the following (as applicable):
-
Certificate of Analysis (CoA) indicating purity and data
-
NMR (¹H/¹³C), MS, and IR spectra
-
Safety Data Sheet (SDS)
-
Batch tracking and quality control records
Purity specifications and detailed analytical results are available on request or at the time of purchase.
ORDERING INFORMATION
-
Packaging: Custom packaging options available
-
Purity Grades: Standard and high‑purity options upon request
-
Technical Support: Application advice and synthetic route consultation
![2-(3-methyl-6-nitro-3H-imidazo[4,5-b]pyridin-2-yl)ethan-1-amine | CAS 1784267-01-1](https://resolvemass.ca/wp-content/uploads/2026/01/2-3-methyl-6-nitro-3H-imidazo45-bpyridin-2-ylethan-1-amine-CAS-1784267-01-1-300x300.png)
Reviews
There are no reviews yet.