PRODUCT OVERVIEW
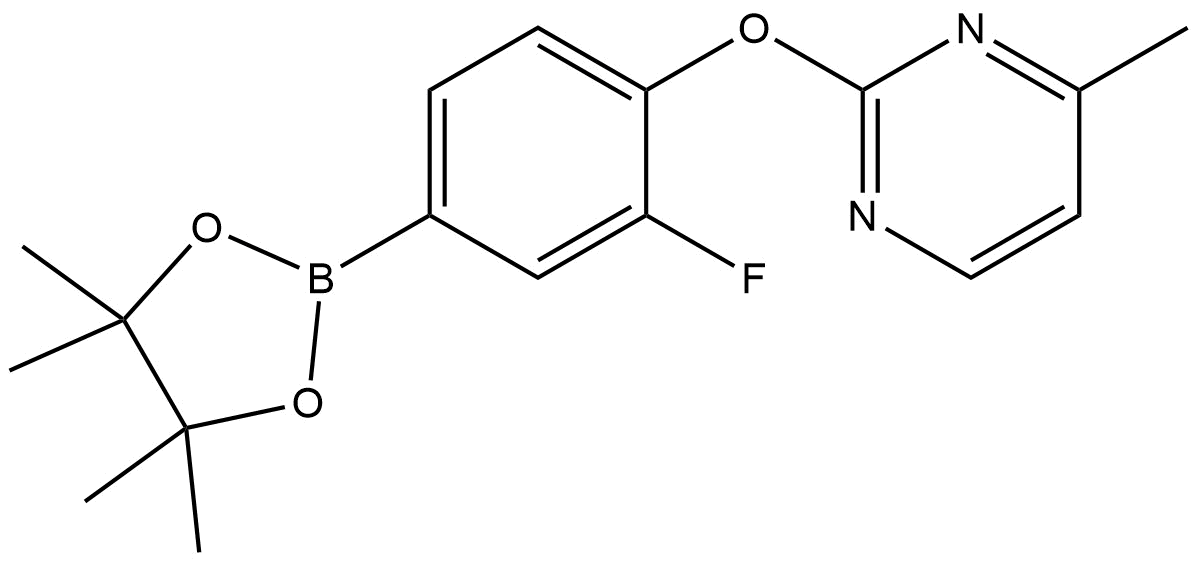
Chemical Name: 2-(2-Fluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenoxy)-4-methylpyrimidine
CAS Number: 2549188-28-3
Molecular Formula: C17H20BFN2O3
Molecular Weight: 330.17 g/mol
2-(2-fluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenoxy)-4-methylpyrimidine | CAS 2549188-28-3 is an advanced synthetic intermediate bearing a boronate ester functional group strategically positioned for use in cross-coupling methodologies. The compound’s design incorporates both a pyrimidine heterocycle and an arylboronate ester—features of high value in medicinal chemistry exploration and screening library construction. Its inclusion of a fluorine substituent adds significant utility for modulation of physicochemical properties, metabolic stability, and binding interactions in drug discovery campaigns.
CHEMICAL STRUCTURE & KEY FEATURES
The molecule consists of the following structural elements:
-
Pyrimidine core: a planar, heterocyclic ring system frequently employed as a scaffold in pharmaceuticals and agrochemicals due to its ability to engage in hydrogen bonding and π-stacking.
-
2-Fluoro-phenoxy linkage: this aryl–oxygen bond provides rigidity and influences electronic properties, with the fluorine substituent altering lipophilicity and potentially enhancing receptor affinity or metabolic stability.
-
Boronate ester functionality (pinacol boronate): a hallmark group for Suzuki-Miyaura cross-coupling, allowing this intermediate to serve as a coupling partner with aryl/vinyl halides to rapidly assemble complex biphenyl or heterobiaryl frameworks.
The pinacol boronate (4,4,5,5-tetramethyl-1,3,2-dioxaborolane) moiety is particularly stable and widely accepted in modern palladium-catalyzed coupling reactions due to its balance of reactivity and handling safety compared to alternative boron reagents.
SYNTHETIC UTILITY
This boronate ester intermediate is ideally positioned for incorporation into high-value compounds where heteroaryl and aryl connectivity is required. Typical transformations include:
-
Suzuki-Miyaura cross-coupling: Enables the formation of C-C bonds with aryl/heteroaryl halides or pseudohalides under palladium catalysis, yielding biaryl and heterobiaryl products.
-
Library diversification: Its use in parallel synthesis approaches can facilitate rapid exploration of structure-activity relationships (SAR) around medicinal targets.
-
Late-stage functionalization: The fluorine substituent and pyrimidine core enable fine-tuning of ADME/pharmacokinetic profiles once coupled into final lead molecules.
Coupling partners may include halogenated heterocycles, substituted phenyl halides, or alkene/vinyl systems depending on the desired target structure. Standard Suzuki conditions employ bases such as potassium carbonate, cesium fluoride, or sodium tert-butoxide in polar aprotic solvents (e.g., dioxane, THF) with Pd(0) or Pd(II) precatalysts.
APPLICATIONS IN RESEARCH of 2-(2-fluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenoxy)-4-methylpyrimidine | CAS 2549188-28-3
Medicinal Chemistry & Drug Discovery:
This compound is primarily used as a building block in the synthesis of medicinally relevant molecules such as kinase inhibitors, antiviral agents, CNS ligands, or other small molecule therapeutics. The fluorine substituent can significantly influence molecular recognition at protein binding sites and often improves pharmacokinetic behavior by reducing oxidative metabolism.
Chemical Biology & Probe Development:
Researchers can incorporate this intermediate into labeled or functionalized probes for target validation, phenotypic screening, or mechanistic studies.
Material Science:
While less common, heterocyclic boronate esters can find utility in functional materials synthesis where electronic properties or fluorescence behavior are tuned through structural modification.
PHYSICAL PROPERTIES
-
Appearance: Off-white to pale solid (typical for aromatic boronate esters)
-
Purity: Analytically verified to ensure suitability for research use in synthesis
-
Solubility: Soluble in common organic solvents such as dichloromethane, THF, and ethyl acetate; limited water solubility typical of boronate esters
(The specific values for melting point or spectral data can be provided upon request or listed in a Certificate of Analysis.)
HANDLING, STORAGE & STABILITY
2-(2-Fluoro-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenoxy)-4-methylpyrimidine should be handled using standard laboratory safety practices. Recommended storage is in a cool, dry environment under inert atmosphere (e.g., nitrogen) to prevent hydrolysis of the boronate ester moiety. Exposure to moisture and prolonged heat should be minimized. Typical shelf life under controlled conditions is consistent with other aromatic pinacol boronate esters.
SAFETY & PRECAUTIONS
As with many fine chemical intermediates, this product is intended for research use only (RUO) and not for human consumption. Consult the Material Safety Data Sheet (MSDS/SDS) before use. Appropriate personal protective equipment (PPE), including gloves, eye protection, and lab coat, should be worn. Avoid inhalation, ingestion, or skin contact. In the event of exposure, follow established emergency procedures.
QUALITY ASSURANCE & ANALYTICAL SUPPORT
At ResolveMass Laboratories Inc., this compound undergoes stringent quality control including:
-
HPLC/UPLC purity assessment
-
NMR (¹H/¹³C) verification
-
Mass spectrometry confirmation
-
Elemental analysis as required
Custom analytical data packages can be provided for project documentation or regulatory filings.
![2-(3-methyl-6-nitro-3H-imidazo[4,5-b]pyridin-2-yl)ethan-1-amine | CAS 1784267-01-1](https://resolvemass.ca/wp-content/uploads/2026/01/2-3-methyl-6-nitro-3H-imidazo45-bpyridin-2-ylethan-1-amine-CAS-1784267-01-1-300x300.png)
Reviews
There are no reviews yet.