PRODUCT OVERVIEW
Compound Name:
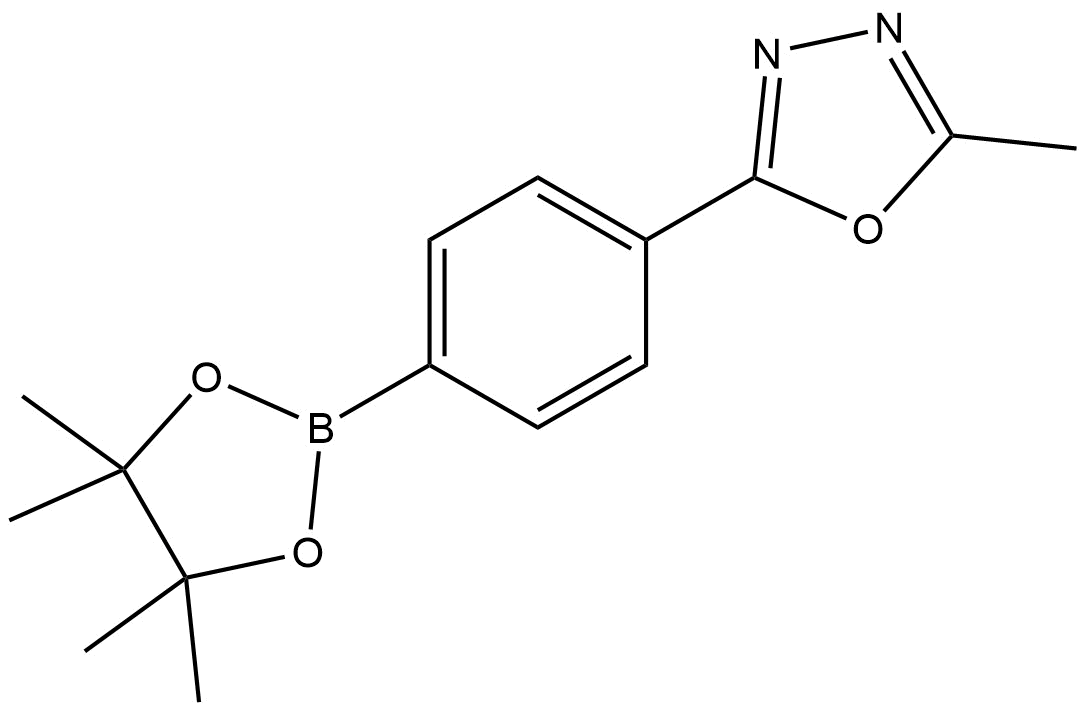
2-Methyl-5-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-1,3,4-oxadiazole
CAS Number:
1056456-24-6
Molecular Formula:
C₁₅H₁₉BN₂O₃
Molecular Weight:
286.14 g/mol
2-methyl-5-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-1,3,4-oxadiazole | CAS 1056456-24-6 is a high-value heterocyclic boronic ester widely used as a research-grade intermediate in organic synthesis, medicinal chemistry, and materials science. The compound combines a chemically stable 1,3,4-oxadiazole core with a pinacol-protected boronic ester, enabling excellent versatility in modern cross-coupling chemistry.
This molecule is designed for laboratories requiring high purity, structural reliability, and reproducible performance in downstream synthetic workflows. ResolveMass Laboratories Inc. supplies this compound for research and development use, supporting early-stage discovery through advanced chemical development programs.
CHEMICAL STRUCTURE & FUNCTIONALITY
The molecular architecture consists of three strategically important components:
-
A 1,3,4-oxadiazole heterocycle, known for its rigidity, electron-deficient character, and stability
-
A para-substituted phenyl ring, acting as a conjugated linker
-
A pinacol boronic ester (1,3,2-dioxaborolane) moiety, which serves as a protected boron functional group
The pinacol ester protects the boron center from premature hydrolysis while maintaining high reactivity under catalytic conditions. This structural feature allows for efficient handling, storage, and integration into multi-step synthesis routes.
The oxadiazole ring contributes to favorable electronic properties, making this compound particularly attractive for applications where molecular stability, polarity control, and heteroatom incorporation are required.
PHYSICOCHEMICAL PROPERTIES of 2-methyl-5-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-1,3,4-oxadiazole | CAS 1056456-24-6
-
Physical State: Solid (typically off-white to pale crystalline powder)
-
Solubility: Soluble in common organic solvents such as THF, dichloromethane, ethyl acetate, and acetonitrile; limited solubility in water
-
Chemical Stability: Stable under standard laboratory conditions when stored properly and protected from moisture
The compound demonstrates good thermal and chemical stability, allowing it to withstand typical reaction conditions used in palladium-catalyzed cross-coupling and related transformations.
SYNTHETIC & RESEARCH APPLICATIONS
Cross-Coupling Chemistry
The boronic ester functionality makes this compound a highly effective Suzuki–Miyaura coupling partner. It readily forms carbon–carbon bonds with aryl and heteroaryl halides, enabling the synthesis of complex biaryl and heteroaryl frameworks. This capability is essential in pharmaceutical development, fine chemical synthesis, and functional material design.
Medicinal Chemistry & Drug Discovery
1,3,4-Oxadiazole motifs are frequently incorporated into drug-like molecules due to their ability to improve metabolic stability, modulate hydrogen bonding, and fine-tune physicochemical properties. This compound is commonly used as a synthetic intermediate for generating diverse analog libraries during hit-to-lead and lead optimization phases.
Advanced Materials & Functional Molecules
Oxadiazole-containing compounds are also explored in materials science, particularly in electronic and optoelectronic research, due to their conjugation and electronic characteristics. The boronic ester functionality further enables post-functionalization, expanding design flexibility.
ANALYTICAL CHARACTERIZATION
Research-grade material is typically characterized using:
-
¹H and ¹³C NMR spectroscopy for structural confirmation
-
Mass spectrometry to verify molecular weight and identity
-
HPLC analysis to confirm purity, commonly ≥98%
These analytical methods ensure consistency, traceability, and suitability for advanced research applications.
HANDLING & STORAGE RECOMMENDATIONS
This compound should be handled in accordance with standard laboratory safety practices:
-
Use appropriate personal protective equipment, including gloves and eye protection
-
Handle in a well-ventilated environment or fume hood
-
Avoid direct contact with skin, eyes, and clothing
For optimal stability, store the material in a tightly sealed container, protected from moisture, heat, and direct light. Refrigerated or cool storage conditions are recommended for long-term preservation.
WHY CHOOSE RESOLVEMASS LABORATORIES INC.
ResolveMass Laboratories Inc. is committed to supplying high-quality research chemicals backed by scientific expertise, rigorous quality standards, and responsive technical support. Each compound is selected and handled with a focus on reliability, reproducibility, and suitability for demanding R&D environments.
Our team understands the needs of medicinal chemists, analytical scientists, and formulation researchers, ensuring that every product supports efficient and confident experimental execution.
SUMMARY
2-Methyl-5-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-1,3,4-oxadiazole (CAS 1056456-24-6) is a versatile, high-purity boronic ester intermediate designed for advanced synthetic chemistry. With its stable oxadiazole core, protected boron functionality, and broad application potential, it represents a valuable tool for modern chemical research.
For technical inquiries, custom synthesis requirements, or bulk supply options, contact ResolveMass Laboratories Inc. through resolvemass.ca.
![2-(3-methyl-6-nitro-3H-imidazo[4,5-b]pyridin-2-yl)ethan-1-amine | CAS 1784267-01-1](https://resolvemass.ca/wp-content/uploads/2026/01/2-3-methyl-6-nitro-3H-imidazo45-bpyridin-2-ylethan-1-amine-CAS-1784267-01-1-300x300.png)
Reviews
There are no reviews yet.