PRODUCT OVERVIEW
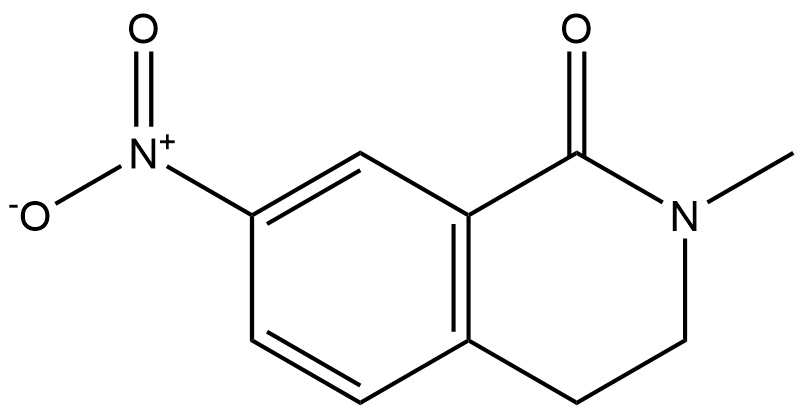
2-methyl-7-nitro-3,4-dihydroisoquinolin-1(2H)-one | CAS 643087-30-3 is a structurally defined heterocyclic organic compound belonging to the dihydroisoquinolinone class. This compound features a partially saturated isoquinoline framework incorporating a lactam functionality at the 1-position, a methyl substituent at the 2-position, and a nitro group at the 7-position. These structural elements collectively confer unique chemical reactivity and make the compound a valuable research intermediate for synthetic and medicinal chemistry applications.
The dihydroisoquinolinone scaffold is widely recognized in pharmaceutical research as a privileged heterocycle due to its rigidity, aromatic character, and compatibility with a broad range of functional group transformations. The incorporation of a nitro substituent further enhances its utility by enabling downstream derivatization into amine-containing analogs, which are frequently encountered in biologically active molecules.
CHEMICAL INFORMATION
-
CAS Number: 643087-30-3
-
Chemical Name: 2-Methyl-7-Nitro-3,4-Dihydroisoquinolin-1(2H)-one
-
Molecular Formula: C₁₀H₁₀N₂O₃
-
Molecular Weight: ~206.20 g/mol
-
Chemical Class: Nitro-substituted dihydroisoquinolinone
-
Functional Groups: Lactam (amide), nitro group, aromatic heterocycle
Structural Characteristics:
The molecule consists of a bicyclic system in which a benzene ring is fused to a partially saturated nitrogen-containing ring. The lactam carbonyl contributes to molecular stability and hydrogen-bonding potential, while the nitro group acts as a strong electron-withdrawing substituent. The methyl group at the 2-position subtly modifies the electronic environment and steric profile of the core, influencing reactivity during synthetic transformations.
PHYSICAL AND ANALYTICAL PROPERTIES
Although compound-specific data may vary by batch and analytical conditions, 2-methyl-7-nitro-3,4-dihydroisoquinolin-1(2H)-one typically exhibits properties consistent with nitro-substituted heterocyclic lactams:
-
Appearance: Solid material under standard laboratory conditions
-
Solubility: Soluble in many common organic solvents such as methanol, ethanol, DMSO, and dichloromethane; limited solubility in water
-
Stability: Chemically stable under recommended storage conditions when protected from moisture and light
Spectroscopic Characteristics:
-
¹H NMR: Aromatic protons appear in the downfield region due to nitro substitution; aliphatic protons from the dihydro ring and methyl group resonate upfield.
-
¹³C NMR: The lactam carbonyl appears significantly downfield, while aromatic carbons adjacent to the nitro group show characteristic deshielding.
-
IR Spectroscopy: Strong absorptions corresponding to the lactam carbonyl and nitro group stretching vibrations are observed.
-
Mass Spectrometry: Molecular ion peak consistent with the expected molecular weight confirms compound identity.
APPLICATIONS & USES
Synthetic Organic Chemistry
2-methyl-7-nitro-3,4-dihydroisoquinolin-1(2H)-one | CAS 643087-30-3 serves as a valuable intermediate in multistep organic synthesis. Its well-defined functional groups allow for predictable reactivity in reduction, substitution, and coupling reactions, making it suitable for advanced synthetic routes.
Medicinal Chemistry Research
The dihydroisoquinolinone core is a well-established pharmacophore in medicinal chemistry. Compounds derived from this scaffold have been investigated for a variety of biological activities when appropriately functionalized. The nitro group at the 7-position offers a strategic handle for conversion into amino derivatives, which can then be further modified to explore structure–activity relationships in drug discovery programs.
Nitro-to-Amine Transformations
One of the most common transformations of this compound involves selective reduction of the nitro group to generate 7-amino-substituted dihydroisoquinolinones. These amine derivatives are highly versatile and can undergo acylation, sulfonylation, urea formation, or heterocycle construction, significantly expanding molecular diversity.
Agrochemical and Fine Chemical Research
Nitro-substituted heterocycles are frequently employed in agrochemical lead discovery and fine chemical synthesis. This compound’s rigid bicyclic framework and modifiable functional groups make it suitable for incorporation into larger molecular architectures designed for biological or industrial applications.
Heterocyclic Scaffold Development
The lactam functionality and fused ring system enable participation in annulation reactions, cross-coupling chemistry, and ring-expansion strategies. As a result, this compound is often used as a platform for constructing more complex heterocyclic systems.
SYNTHETIC FLEXIBILITY
The chemical architecture of 2-methyl-7-nitro-3,4-dihydroisoquinolin-1(2H)-one supports a wide range of transformations:
-
Catalytic hydrogenation or chemical reduction of the nitro group
-
Functionalization at the nitrogen or aromatic ring positions
-
Integration into multicomponent or cascade reaction sequences
-
Use as a precursor for bioisosteric analog development
This flexibility makes it especially attractive to researchers focused on rapid lead optimization and scaffold diversification.
QUALITY ASSURANCE
At ResolveMass Laboratories Inc., 2-methyl-7-nitro-3,4-dihydroisoquinolin-1(2H)-one (CAS 643087-30-3) is supplied as a research-grade material with strict quality control standards. Each batch is produced and verified to meet high purity requirements suitable for demanding synthetic and medicinal chemistry workflows. Analytical documentation, including Certificates of Analysis, is available upon request.
SAFETY & HANDLING
This compound is intended strictly for research and development use. It is not approved for human or veterinary applications.
-
Handle using appropriate laboratory PPE
-
Avoid inhalation, ingestion, or direct skin contact
-
Use in a well-ventilated area or fume hood
-
Store in a tightly sealed container under recommended conditions
![2-(3-methyl-6-nitro-3H-imidazo[4,5-b]pyridin-2-yl)ethan-1-amine | CAS 1784267-01-1](https://resolvemass.ca/wp-content/uploads/2026/01/2-3-methyl-6-nitro-3H-imidazo45-bpyridin-2-ylethan-1-amine-CAS-1784267-01-1-300x300.png)
Reviews
There are no reviews yet.