OVERVIEW
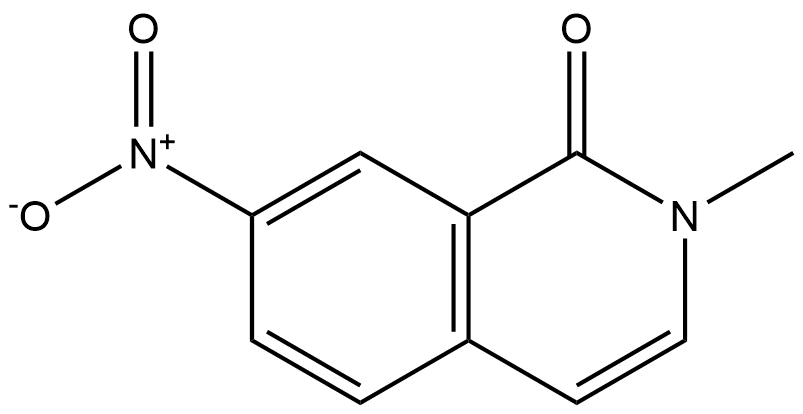
2-methyl-7-nitroisoquinolin-1(2H)-one | CAS 54931-59-8 is a structurally complex nitrogen-containing heterocycle belonging to the isoquinolinone family. Isoquinolinone derivatives are widely recognized in chemical research due to their relevance in medicinal chemistry, synthetic organic chemistry, and advanced intermediate development. The presence of both a nitro functional group and a lactam (1-one) moiety makes this compound particularly valuable as a versatile synthetic intermediate.
This molecule features a fused bicyclic aromatic system with a methyl substitution at the 2-position and a nitro group at the 7-position. Such substitution patterns significantly influence the compound’s electronic distribution, reactivity, and potential for downstream functionalization. As a result, 2-methyl-7-nitroisoquinolin-1(2H)-one is commonly explored in research workflows requiring structurally rigid, electronically tunable heterocycles.
ResolveMass Laboratories Inc. supplies this compound for research and development applications, ensuring consistent quality and suitability for advanced synthetic programs.
CHEMICAL IDENTITY & STRUCTURAL FEATURES
-
CAS Number: 54931-59-8
-
Chemical Name: 2-Methyl-7-nitroisoquinolin-1(2H)-one
-
Chemical Class: Isoquinolinone heterocycles
-
Core Functional Groups:
-
Nitro group (–NO₂)
-
Lactam carbonyl (–C=O)
-
Aromatic heterocyclic ring
-
Alkyl (methyl) substituent
-
The isoquinolinone framework provides a rigid and planar aromatic system, while the nitro group introduces strong electron-withdrawing properties. This combination enhances the compound’s utility in selective reactions such as reduction, nucleophilic substitution, and heterocycle derivatization. The lactam functionality also allows hydrogen bonding and contributes to the compound’s stability under controlled laboratory conditions.
PHYSICOCHEMICAL PROPERTIES
2-methyl-7-nitroisoquinolin-1(2H)-one | CAS 54931-59-8 typically appears as a light yellow to off-white crystalline solid, characteristic of nitro-substituted aromatic compounds. It demonstrates:
-
Moderate polarity, driven by the nitro and carbonyl groups
-
Limited aqueous solubility, with improved solubility in polar organic solvents
-
Thermal stability appropriate for standard laboratory synthesis conditions
The electronic influence of the nitro group affects both solubility and reactivity, making this compound suitable for controlled transformation steps in multi-stage synthesis.
SYNTHETIC RELEVANCE
From a synthetic chemistry perspective, this compound serves as a strategic intermediate in the preparation of more complex isoquinoline-based structures. Common synthetic applications include:
-
Nitro group reduction to yield amino-substituted isoquinolinones
-
Functional group interconversion at the lactam site
-
Cross-coupling and annulation reactions following appropriate activation
Nitro groups are particularly valuable in synthetic chemistry due to their predictable transformation pathways. Reduction of the nitro group enables access to amine derivatives, which can subsequently undergo acylation, sulfonylation, or heterocycle fusion reactions. This makes 2-methyl-7-nitroisoquinolin-1(2H)-one a flexible building block in both exploratory and scale-up chemistry.
APPLICATIONS of 2-methyl-7-nitroisoquinolin-1(2H)-one | CAS 54931-59-8
Pharmaceutical & Medicinal Chemistry Research
Isoquinolinone scaffolds are frequently investigated for biological relevance. While this compound itself is primarily used as an intermediate, its derivatives may contribute to the development of molecules with potential pharmacological activity. Researchers value the scaffold for its structural rigidity and capacity for targeted modification.
Chemical Intermediate for Advanced Synthesis
This compound is well-suited for inclusion in heterocyclic compound libraries, particularly where nitro-to-amine transformations are required. Its defined substitution pattern allows chemists to explore structure–activity relationships efficiently.
Agrochemical & Specialty Chemical R&D
Nitro heterocycles are often utilized in early-stage agrochemical and specialty material research due to their electronic properties and stability. 2-Methyl-7-nitroisoquinolin-1(2H)-one can function as a precursor for compounds with tailored physicochemical profiles.
ANALYTICAL CHARACTERIZATION
For research and quality assurance purposes, this compound is commonly characterized using:
-
¹H and ¹³C NMR spectroscopy for structural confirmation
-
Mass spectrometry (MS) for molecular weight verification
-
Infrared spectroscopy (IR) to confirm nitro and carbonyl functionalities
-
HPLC or LC-MS for purity assessment
These analytical techniques ensure reproducibility and confidence in downstream applications.
SAFETY & HANDLING CONSIDERATIONS
As with most nitro-substituted aromatic compounds, appropriate laboratory precautions should be followed:
-
Use personal protective equipment including gloves, eye protection, and lab coats
-
Handle in a well-ventilated environment
-
Avoid exposure to excessive heat or strong reducing agents unless under controlled reaction conditions
This compound is intended for research use only and should be handled by trained professionals familiar with heterocyclic and nitro compound chemistry.
WHY CHOOSE RESOLVEMASS LABORATORIES INC.
ResolveMass Laboratories Inc. is committed to supplying high-quality research chemicals backed by scientific expertise, analytical rigor, and dependable quality standards. Our compounds are sourced and verified to meet the needs of pharmaceutical, chemical, and academic research laboratories worldwide.
![5-bromo-6-iodo-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine | CAS 2549186-23-2](https://resolvemass.ca/wp-content/uploads/2026/01/5-bromo-6-iodo-7-methyl-7H-pyrrolo23-dpyrimidin-4-amineCAS-2549186-23-2-300x300.png)
Reviews
There are no reviews yet.