OVERVIEW
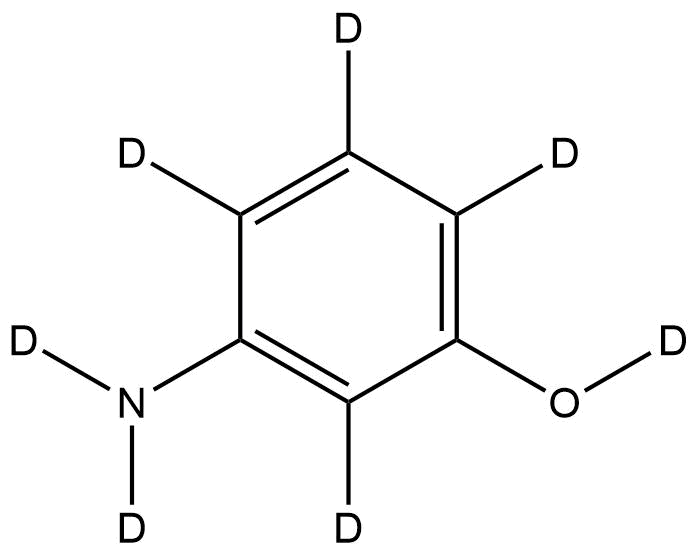
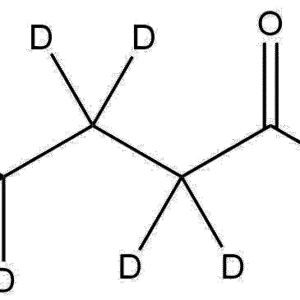
3-Aminophenol-d₇ is a fully deuterated analog of 3-aminophenol, in which all exchangeable hydrogen atoms are replaced with deuterium, resulting in a +7 Da isotopic mass shift. This compound retains the core structure and reactivity of 3-aminophenol while serving as a stable isotope-labeled compound for advanced analytical and research applications. It is commonly used in mass spectrometry, pharmaceutical research, and organic synthesis to study reaction mechanisms, quantify trace levels of unlabeled compounds, and evaluate kinetic isotope effects.
3-Aminophenol (meta-aminophenol) is a key intermediate in the synthesis of pharmaceuticals, dyes, antioxidants, and hair colorants. The deuterated variant, 3-aminophenol-d₇, provides valuable analytical precision for quantification and tracing due to its isotopic distinction from the non-labeled compound, without any alteration to its chemical behavior.
CHEMICAL INFORMATION
-
Name: 3-Aminophenol-d₇
-
Molecular Formula: C₆D₇NO
-
Molecular Weight: 116.17 g/mol
-
CAS Number: 591-27-5
-
Isotopic Enrichment: ≥ 98 atom % D
-
Chemical Class: Deuterated aromatic amine and phenol
-
Stability: Stable under inert and dry conditions; may oxidize upon prolonged air exposure
APPLICATIONS of 3-Aminophenol-d7 | CAS 591-27-5
-
Analytical Chemistry & Isotopic Labeling:
3-Aminophenol-d₇ serves as a stable isotope-labeled internal standard in LC-MS and GC-MS for the quantification of 3-aminophenol and its derivatives in environmental, biological, and industrial matrices. -
Pharmaceutical and Biochemical Research:
Used to trace metabolic pathways and biotransformation processes involving aromatic amines and phenolic intermediates. It supports drug metabolism studies and kinetic isotope effect evaluations in biochemical systems. -
Synthetic Organic Chemistry:
Acts as a precursor for synthesizing deuterated pharmaceuticals, dyes, and fine chemicals, particularly where controlled isotopic labeling is required to study chemical transformations or reaction rates. -
Kinetic and Mechanistic Studies:
Deuterium substitution allows researchers to investigate hydrogen-transfer reactions, oxidation–reduction mechanisms, and substitution kinetics, providing insight into reaction dynamics through isotopic effects. -
Spectroscopic and NMR Applications:
Deuteration eliminates interfering proton signals in ¹H NMR, simplifying spectra and enabling more precise interpretation of complex molecular systems. The compound is also suitable for ²H NMR analyses.
ADVANTAGES of 3-Aminophenol-d7 | CAS 591-27-5
-
High isotopic enrichment ensures reliable mass spectral resolution.
-
Chemically and physically identical to non-deuterated 3-aminophenol.
-
Provides consistent results in quantitative isotope dilution methods.
-
Enhances structural clarity in NMR and IR spectroscopy.
-
Ideal for studying proton exchange, tautomerism, and intramolecular dynamics.
HANDLING
-
Hazards: May cause irritation to skin, eyes, and respiratory tract; avoid contact and inhalation.
-
Handling Precautions: Use gloves, lab coat, and eye protection. Avoid exposure to air and light.
-
Storage: Keep container tightly closed in a cool, dry, and inert atmosphere.
-
Disposal: Dispose of according to institutional and regional regulations for organic waste.
QUALITY & SPECIFICATION
-
Chemical Purity: ≥ 98 %
-
Isotopic Enrichment: ≥ 98 atom % D
-
Water Content: ≤ 0.5 %
-
Analytical Verification: Confirmed by ¹H NMR (absence of proton signals), ²H NMR, LC-MS, and IR spectroscopy
-
Appearance: White to off-white crystalline powder
-
Documentation: Supplied with Certificate of Analysis specifying isotopic enrichment and purity
SUMMARY
3-Aminophenol-d₇ (CAS 591-27-5) is a high-purity, stable isotope-labeled analog of 3-aminophenol, offering exceptional analytical precision and isotopic reliability. It is an indispensable reagent in mass spectrometry, pharmaceutical metabolism, and mechanistic organic chemistry. By maintaining chemical equivalence with its non-deuterated counterpart and providing robust isotopic differentiation, 3-aminophenol-d₇ enhances the accuracy, reproducibility, and interpretability of modern analytical and research methodologies.
Learn more about,
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Availability of All the Deuterated Chemicals at ResolveMass Laboratories Inc.
ResolveMass Laboratories: Leading Deuterated Chemical Synthesis Company in the United States.
Deuterated Internal Standards for LC-MS: Selection & Custom Synthesis
How to Choose the Right Deuterated Labelled Chemical Synthesis Company in Canada
How to Choose the Right Deuterium Labelled Compounds Supplier for Your Lab
Deuterium-Labelled Compounds — Synthesis, Applications & Ordering
Reviews
There are no reviews yet.