PRODUCT NAME
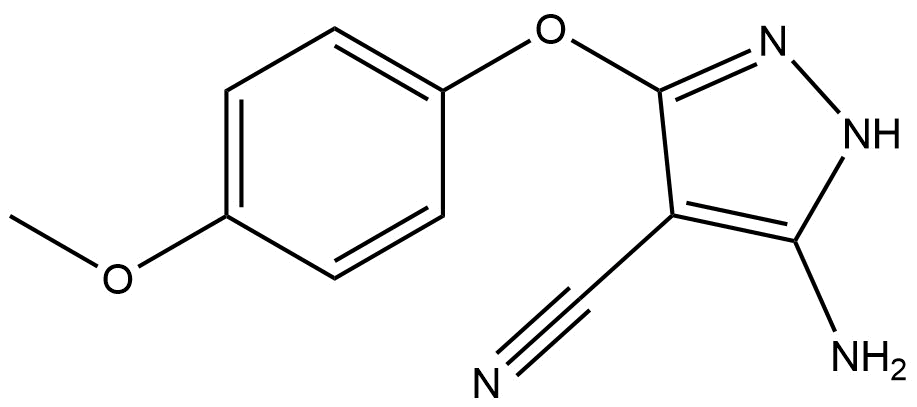
5-amino-3-(4-methoxyphenoxy)-1H-pyrazole-4-carbonitrile | CAS 117174-18-2
OVERVIEW
5-amino-3-(4-methoxyphenoxy)-1H-pyrazole-4-carbonitrile | CAS 117174-18-2 is a specialized heterocyclic organic compound widely used as a research and synthetic intermediate in pharmaceutical, agrochemical, and advanced chemical research. Structurally, the molecule is built on a pyrazole core substituted with an amino group, a carbonitrile moiety, and a para-methoxyphenoxy aromatic ether, making it a chemically versatile and functionally rich compound.
Pyrazole derivatives are highly valued in modern chemistry due to their stability, tunable electronic properties, and compatibility with a broad range of chemical transformations. The presence of multiple reactive functional groups within this molecule allows it to serve as a key building block for the synthesis of structurally complex molecules, particularly those requiring nitrogen-rich heterocycles.
At ResolveMass Laboratories Inc., this compound is supplied for research use only and is intended for qualified professionals engaged in discovery chemistry, medicinal chemistry programs, and synthetic method development.
CHEMICAL & STRUCTURAL FEATURES
The molecular framework of 5-amino-3-(4-methoxyphenoxy)-1H-pyrazole-4-carbonitrile combines both aromatic and heteroaromatic systems, contributing to its chemical robustness and adaptability.
Key structural elements include:
-
Pyrazole ring system, a five-membered heterocycle containing two nitrogen atoms
-
Amino group (-NH₂) at the 5-position, offering hydrogen-bonding capability and synthetic reactivity
-
Carbonitrile group (-C≡N) at the 4-position, enabling further derivatization and ring-forming reactions
-
4-Methoxyphenoxy substituent, introducing aromatic character and electronic modulation
This combination of polar and non-polar functionalities supports diverse reaction pathways, making the compound suitable for both linear synthesis and scaffold modification strategies.
PHYSICAL & CHEMICAL CHARACTERISTICS
While batch-specific analytical values are provided in the Certificate of Analysis, compounds of this class typically exhibit the following general characteristics:
-
Solid appearance at room temperature
-
Good stability under standard laboratory conditions
-
Low aqueous solubility with improved solubility in common organic solvents such as DMSO, DMF, methanol, and acetonitrile
-
Thermal stability consistent with substituted heteroaromatic compounds
The balanced polarity of the molecule allows for compatibility with both solution-phase synthesis and analytical characterization techniques such as HPLC, LC-MS, and NMR.
APPLICATIONS of 5-amino-3-(4-methoxyphenoxy)-1H-pyrazole-4-carbonitrile | CAS 117174-18-2
Medicinal Chemistry & Drug Discovery
Pyrazole-based compounds are widely investigated for their presence in pharmacologically active molecules. 5-Amino-3-(4-methoxyphenoxy)-1H-pyrazole-4-carbonitrile serves as a valuable intermediate in medicinal chemistry programs focused on the design and optimization of small-molecule therapeutics. The amino and nitrile functionalities provide multiple points for structural modification, enabling the generation of analogues for structure–activity relationship (SAR) studies.
Agrochemical & Crop Protection Research
Heterocyclic compounds containing pyrazole cores are frequently explored in agrochemical research due to their biological stability and functional diversity. This compound can be used in early-stage synthesis programs targeting novel herbicidal, fungicidal, or pesticidal agents.
Synthetic & Heterocyclic Chemistry
The compound is well suited for advanced heterocyclic synthesis, including cyclization reactions, nucleophilic substitutions, and functional group interconversions. The nitrile group, in particular, allows access to amides, heterocycles, and fused ring systems, expanding its utility in complex molecule construction.
Chemical Library Development
In combinatorial and parallel synthesis workflows, this molecule acts as a reliable scaffold for expanding screening libraries. Its structural features allow chemists to rapidly generate derivative compounds with varied physicochemical profiles.
QUALITY & ANALYTICAL STANDARDS
ResolveMass Laboratories Inc. is committed to supplying high-quality research chemicals that meet the rigorous demands of scientific research. Each batch of 5-amino-3-(4-methoxyphenoxy)-1H-pyrazole-4-carbonitrile is manufactured and tested under controlled conditions.
Typical quality attributes include:
-
High chemical purity suitable for R&D applications
-
Verification using analytical techniques such as HPLC, LC-MS, and NMR
-
Batch-specific Certificate of Analysis (CoA) provided with every order
Custom purity requirements and analytical documentation may be supported upon request.
WHY CHOOSE RESOLVEMASS LABORATORIES INC.
ResolveMass Laboratories Inc. is a trusted supplier of specialty chemicals and research intermediates, supporting innovation across pharmaceutical, agrochemical, and advanced materials research. Our commitment to quality, documentation, and scientific reliability ensures that researchers receive materials they can confidently integrate into their workflows.
![4-(4-amino-1H-pyrazolo[3,4-d]pyrimidin-3-yl)phenol | CAS 1293915-57-7](https://resolvemass.ca/wp-content/uploads/2026/01/4-4-amino-1H-pyrazolo34-dpyrimidin-3-ylphenol-CAS-1293915-57-7-2.png)
Reviews
There are no reviews yet.