PRODUCT OVERVIEW
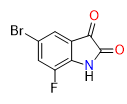
5-bromo-7-fluoroindoline-2,3-dione | CAS 74830-75-8 is a synthetically significant halogenated isatin derivative. It features a unique substitution pattern — a bromine atom at the 5-position and a fluorine atom at the 7-position of the indoline-2,3-dione core. This combination of functional groups imparts distinct electronic and steric attributes, making it an important intermediate in organic synthesis and medicinal chemistry research.
As an advanced intermediate, 5-bromo-7-fluoroindoline-2,3-dione serves as a valuable building block for the design and synthesis of heterocyclic compounds, bioactive small molecules, and pharmaceutical derivatives. Its halogen substituents facilitate cross-coupling reactions, nucleophilic aromatic substitution, and can direct selective functionalization pathways.
CHEMICAL STRUCTURE & IDENTIFICATION
-
Chemical Name: 5-Bromo-7-fluoroindoline-2,3-dione
-
CAS Number: 74830-75-8
-
Molecular Formula: C8H3BrFNO2
-
Molecular Weight: 244.02 g/mol
-
Functional Groups: Halogenated indoline-2,3-dione core with bromine and fluorine substituents
The compound’s indoline-2,3-dione core (also known as isatin) is a well recognized privileged scaffold in organic chemistry. The introduction of bromine and fluorine enhances reactivity and enables a range of synthetic transformations that are useful in constructing more complex motifs.
KEY PHYSICAL & CHEMICAL PROPERTIES
| Property | Typical Specification |
|---|---|
| Appearance | Off-white to light tan solid |
| Purity | ≥99% (by HPLC) |
| Melting Point | Typically >200 °C (decomposes) |
| Solubility | Soluble in DMSO, DMF; moderate in acetone |
| Stability | Stable under recommended storage conditions |
5-Bromo-7-fluoroindoline-2,3-dione demonstrates solid thermal stability up to its high melting point, making it amenable to various reaction conditions used in synthetic laboratories. The presence of halogen substituents also influences its polarity and reactivity.
SYNTHETIC APPLICATIONS
HALOGEN-MEDIATED CROSS-COUPLING
The bromine substituent at position 5 is a prime handle for palladium-catalyzed cross-coupling reactions such as:
-
Suzuki–Miyaura coupling
-
Stille coupling
-
Negishi coupling
These reactions enable the installation of aryl, heteroaryl, or alkyl groups, allowing the construction of diversified libraries of indoline derivatives with potential biological activities.
FLUORINE-ENHANCED REACTIVITY
The fluorine at the 7-position contributes to altered electronic density in the ring and can:
-
Increase metabolic stability in derived compounds.
-
Modify binding interactions in bioactive candidates.
-
Serve as a directing group in regioselective functionalizations.
NUCLEOPHILIC AROMATIC SUBSTITUTION (SNAr)
Under appropriate conditions, the electron-deficient aromatic ring, activated by fluorine and the adjacent carbonyl groups, can engage in nucleophilic aromatic substitution, especially with soft nucleophiles like amines and thiols. This enables access to 7-substituted isatin analogs.
BUILDING BLOCK IN DRUG DISCOVERY
The combined presence of electron-withdrawing halogens and the indoline-2,3-dione scaffold makes this compound particularly useful in medicinal chemistry for designing:
-
Enzyme inhibitors
-
Receptor ligands
-
Fluorinated heterocyclic libraries
Fluorination is a common strategy in drug design to enhance pharmacokinetic properties, and the 7-fluoro motif here provides a strategic position for modulation.
RESEARCH & DEVELOPMENT USES
Researchers in organic synthesis, medicinal chemistry, and materials science leverage 5-bromo-7-fluoroindoline-2,3-dione in multiple contexts:
-
Synthesis of fluorinated bioactive compounds: Enables rapid diversification using cross-coupling and substitution.
-
Fragment-based drug discovery: Acts as a core scaffold that can be elaborated with diverse functional groups.
-
Method development: Used as a substrate to probe new catalytic systems, especially in halogen-selective transformations.
-
Structure–activity relationship (SAR) studies: Facilitates systematic variation of substituents to optimize biological properties.
QUALITY & ANALYTICAL CHARACTERIZATION
At ResolveMass Laboratories Inc., quality is a cornerstone of our offerings. 5-Bromo-7-fluoroindoline-2,3-dione (CAS 74830-75-8) is supplied with:
-
High purity (≥99%), verified by HPLC or GC where applicable.
-
Confirmatory spectra, including NMR (1H/13C) and MS, to validate identity.
-
Batch-specific certificates indicating analytical results and traceability.
Our analytical protocols ensure consistent performance in research workflows.
SAFETY & HANDLING
This compound should be handled in accordance with standard laboratory safety practices:
-
Use appropriate personal protective equipment (PPE).
-
Conduct manipulations in a fume hood.
-
Avoid inhalation, ingestion, or skin contact.
-
Store in a cool, dry place, away from strong oxidizers.
Please consult the Safety Data Sheet (SDS) for detailed hazard and handling information.
CONCLUSION
5-Bromo-7-fluoroindoline-2,3-dione | CAS 74830-75-8 is a versatile, high-purity intermediate ideal for advanced synthetic chemistry and drug discovery applications. Its strategic halogenation pattern enables diverse functionalization routes, making it a valuable reagent for researchers seeking fluorinated heterocyclic frameworks.
For inquiries, bulk availability, and custom quantities, contact ResolveMass Laboratories Inc. directly through our product page.
Reviews
There are no reviews yet.