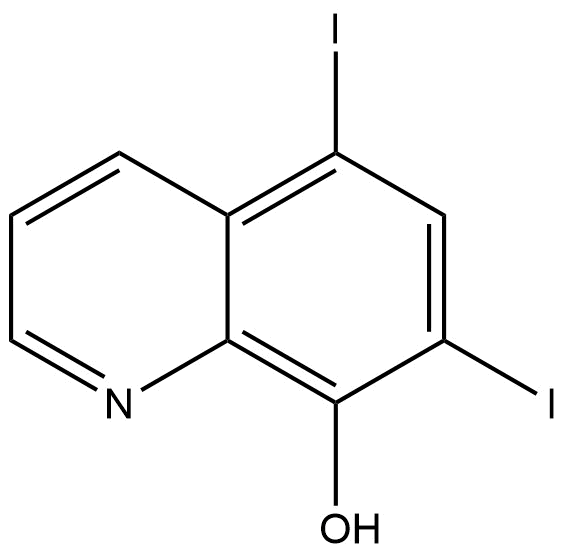
5,7-DIIODO-8-HYDROXYQUINOLINE | CAS 83-73-8
OVERVIEW
5,7-Diiodo-8-hydroxyquinoline | CAS 83-73-8 , also known as iodoquinol, is a halogenated quinoline derivative widely recognized for its distinctive chemical structure and functional versatility. With the molecular formula C₉H₅I₂NO and a molecular weight of approximately 396.95 g/mol, this compound belongs to the 8-hydroxyquinoline family, a class known for metal-binding properties and broad applicability in pharmaceutical and analytical research.
The molecule is characterized by iodine substitutions at the 5 and 7 positions of the quinoline ring and a hydroxyl group at the 8 position. This structural configuration contributes to enhanced lipophilicity, chemical stability, and biological activity when compared to non-halogenated analogs. Due to these attributes, 5,7-Diiodo-8-hydroxyquinoline continues to be of interest across multiple research domains, including medicinal chemistry, bioanalysis, and coordination chemistry.
CHEMICAL IDENTITY
-
Chemical Name: 5,7-Diiodo-8-hydroxyquinoline
-
CAS Number: 83-73-8
-
Synonyms: Iodoquinol, Diiodohydroxyquinoline, 5,7-Diiodoquinolin-8-ol
-
Molecular Formula: C₉H₅I₂NO
-
Molecular Weight: 396.95 g/mol
PHYSICAL AND CHEMICAL PROPERTIES
5,7-Diiodo-8-hydroxyquinoline | CAS 83-73-8 is typically supplied as a light yellow to tan crystalline powder. It exhibits high thermal stability under ambient laboratory conditions, with decomposition occurring at elevated temperatures. The compound shows very low vapor pressure, making it suitable for controlled laboratory handling and storage.
The compound is practically insoluble in water but demonstrates limited solubility in certain organic solvents such as alcohols and ethers. Its relatively high log P value reflects a strong lipophilic character, which plays an important role in both formulation considerations and biological interactions. The presence of heavy iodine atoms significantly influences its density and refractive index, further distinguishing it from other hydroxyquinoline derivatives.
STRUCTURAL CHARACTERISTICS
As a substituted quinoline, 5,7-Diiodo-8-hydroxyquinoline contains a fused aromatic heterocycle with a nitrogen atom in the ring system. The hydroxyl group at the 8 position enables chelating behavior, allowing the compound to form stable complexes with a range of metal ions. The iodine substituents enhance electron distribution across the aromatic system, which can influence reactivity, binding affinity, and analytical performance.
This combination of heterocyclic structure and halogenation makes the compound particularly attractive for advanced research involving metal coordination, enzyme interaction studies, and structure–activity relationship investigations.
APPLICATIONS IN RESEARCH AND INDUSTRY
Pharmaceutical and Medicinal Chemistry Research
5,7-Diiodo-8-hydroxyquinoline has a long history of investigation in pharmaceutical research, particularly in studies related to antimicrobial and antiparasitic activity. While its historical therapeutic use is well documented, it remains relevant today as a reference compound, research standard, and synthetic intermediate in the development of new quinoline-based drug candidates.
Researchers utilize this compound to explore structure-activity relationships, iodine-substituted heterocycles, and halogen effects on biological systems.
Analytical Chemistry and Bioanalysis
In analytical laboratories, this compound is valued for its metal-binding capability, which enables its use in complexation-based analytical techniques. It has been applied in spectrophotometric and extraction-based methods for metal ion determination and serves as a useful reagent in coordination chemistry studies.
Its predictable chemical behavior makes it suitable for method development, validation work, and comparative analytical studies.
Materials and Coordination Chemistry
The chelating properties of 5,7-Diiodo-8-hydroxyquinoline support its use in materials science research, particularly in the synthesis of metal-organic complexes. Such complexes are investigated for their optical, electronic, and functional properties, including potential applications in sensing technologies and advanced material systems.
QUALITY AND RESEARCH STANDARDS
At ResolveMass Laboratories Inc., 5,7-Diiodo-8-hydroxyquinoline is intended strictly for research and laboratory use. The compound is suitable for applications requiring consistent chemical identity, reproducibility, and performance in R&D environments. Quality control processes ensure alignment with defined analytical specifications, supporting reliable experimental outcomes.
SUMMARY
5,7-Diiodo-8-hydroxyquinoline (CAS 83-73-8) is a high-value heterocyclic compound with proven relevance in pharmaceutical research, analytical chemistry, and materials science. Its unique iodine-substituted quinoline structure, combined with metal-chelating capability and chemical stability, makes it an essential tool for advanced scientific investigations. ResolveMass Laboratories Inc. supplies this compound to meet the evolving needs of research professionals seeking dependable and well-characterized chemical materials.
![4-(4-amino-1H-pyrazolo[3,4-d]pyrimidin-3-yl)phenol | CAS 1293915-57-7](https://resolvemass.ca/wp-content/uploads/2026/01/4-4-amino-1H-pyrazolo34-dpyrimidin-3-ylphenol-CAS-1293915-57-7-2.png)
![6-bromo-5-iodo-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine | CAS 2894018-46-1](https://resolvemass.ca/wp-content/uploads/2026/01/6-bromo-5-iodo-7-methyl-7H-pyrrolo23-dpyrimidin-4-amineCAS-2894018-46-1-300x300.png)
Reviews
There are no reviews yet.