PRODUCT OVERVIEW
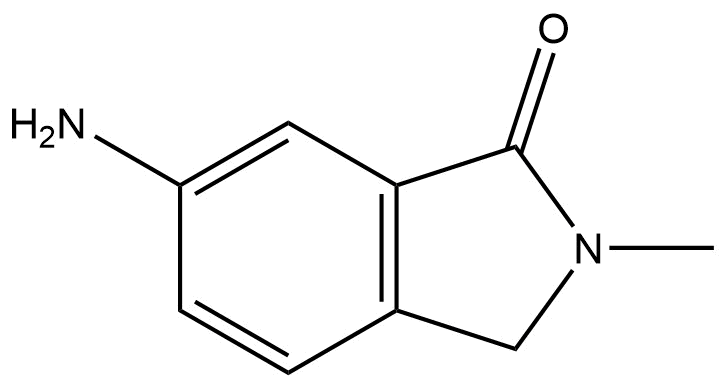
Product Name: 6‑Amino‑2‑methylisoindolin‑1‑one
CAS Number: 69189‑26-0
Molecular Formula: C₉H₁₀N₂O
Molecular Weight: 162.19 g/mol
6-amino-2-methylisoindolin-1-one | CAS 69189-26-0 is a heterocyclic organic compound featuring a fused benzene and pyrrolidinone (isoindolinone) ring system, functionalized with an amino group at the 6‑position and a methyl group at the 2‑position. Its unique combination of structural elements makes it a versatile intermediate for chemical synthesis and a valuable building block in medicinal chemistry.
CHEMICAL STRUCTURE AND CLASSIFICATION
This compound belongs to the isoindolinone class of heterocycles, characterized by a benzene ring fused to a five-membered nitrogen-containing lactam ring. The primary amine at the 6‑position provides a reactive site for functionalization, while the carbonyl group at the 1‑position (lactam) enables nucleophilic addition and other transformations. The methyl group at the 2‑position influences steric and electronic properties, impacting reactivity and interaction with other reagents. These features collectively make 6‑Amino‑2‑methylisoindolin‑1‑one an important intermediate in both research and industrial chemistry.
PHYSICAL AND CHEMICAL PROPERTIES
6‑Amino‑2‑methylisoindolin‑1‑one is typically supplied as an off-white crystalline powder. It has good solubility in polar organic solvents such as methanol and ethanol, while water solubility is limited. The compound is stable under normal laboratory conditions when stored in a cool, dry place, and is resistant to decomposition in the absence of strong oxidants. Standard storage temperatures are typically between 2–8 °C for long-term stability. Analytical methods such as HPLC, NMR, and mass spectrometry are commonly used to confirm purity and identity.
SYNTHESIS AND MANUFACTURE
The synthesis of 6‑Amino‑2‑methylisoindolin‑1‑one generally involves multi-step processes, beginning with the formation of the isoindolinone core through cyclization of aromatic precursors. The methyl group at the 2‑position is introduced via alkylation reactions, while the amino group at the 6‑position is installed through substitution or reductive amination strategies. Alternative methods may include transition-metal-catalyzed reactions or multi-component syntheses that allow efficient construction of the heterocyclic framework. Scale-up synthesis emphasizes controlled reaction conditions, optimized catalysts, and efficient purification to achieve high-purity product suitable for research or as an intermediate.
CHEMICAL REACTIVITY AND TRANSFORMATIONS
The compound’s functional groups enable a wide range of chemical transformations:
-
Amino Group Reactivity: The primary amine allows acylation, alkylation, reductive amination, and coupling reactions, enabling synthesis of diverse derivatives.
-
Lactam Carbonyl Chemistry: The carbonyl group can undergo nucleophilic additions, reductions, and other reactions to modify the heterocyclic core.
-
Aromatic Substitution: Positions on the benzene ring can participate in electrophilic substitution, allowing further chemical modification and diversification.
This combination of reactivity makes 6‑Amino‑2‑methylisoindolin‑1‑one a flexible platform for creating more complex molecules for research and development.
APPLICATIONS of 6-amino-2-methylisoindolin-1-one | CAS 69189-26-0
Medicinal Chemistry & Drug Discovery
6‑Amino‑2‑methylisoindolin‑1‑one serves as a versatile intermediate for the synthesis of heterocyclic compounds with potential therapeutic applications. Its amino and lactam functionalities allow for attachment of pharmacologically active groups, supporting drug discovery and design.
Organic Synthesis
It is widely used in multi-step synthetic pathways, facilitating the construction of complex molecular architectures. Its structural flexibility makes it useful for generating compound libraries for screening and chemical research.
Materials and Chemical Research
Derivatives of isoindolinones are explored in advanced molecular materials, fluorescent studies, and photochemical applications, highlighting the compound’s versatility beyond conventional synthesis.
Academic and Fundamental Studies
The compound is often used in mechanistic and structural studies to understand heterocycle reactivity, stability, and functional group transformations in a laboratory setting.
CONCLUSION
6‑Amino‑2‑methylisoindolin‑1‑one (CAS 69189‑26-0) is a chemically robust and synthetically versatile heterocyclic compound. Its defined structure, reactive functional groups, and broad utility in chemical synthesis, medicinal chemistry, and advanced research make it an essential intermediate for laboratories and industrial applications. It is a reliable and high-value building block for creating diverse molecular frameworks and exploring new chemical transformations.
![3-(3-fluoro-4-isopropoxyphenyl)-1H-pyrazolo[3,4-d]pyrimidin-4-amine | CAS 1408087-64-8](https://resolvemass.ca/wp-content/uploads/2026/01/3-3-fluoro-4-isopropoxyphenyl-1H-pyrazolo34-dpyrimidin-4-amineCAS-1408087-64-8-300x300.png)
![2-(3-methyl-6-nitro-3H-imidazo[4,5-b]pyridin-2-yl)ethan-1-amine | CAS 1784267-01-1](https://resolvemass.ca/wp-content/uploads/2026/01/2-3-methyl-6-nitro-3H-imidazo45-bpyridin-2-ylethan-1-amine-CAS-1784267-01-1-300x300.png)
Reviews
There are no reviews yet.