OVERVIEW
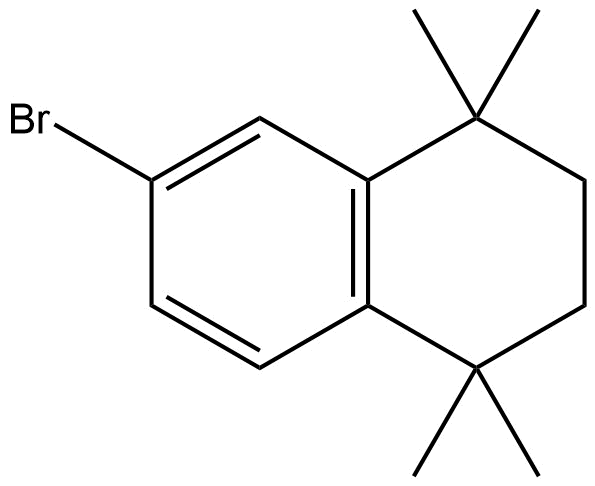
6-Bromo-1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthalene | CAS 27452-17-1 is a high-purity brominated tetrahydronaphthalene derivative with unique structural and chemical properties that make it valuable in synthetic organic chemistry. This compound features a bromine atom at the 6-position of a tetrahydronaphthalene core, combined with sterically bulky 1,1,4,4-tetramethyl substitutions, imparting distinctive reactivity and stability. Its molecular architecture enables it to serve as a versatile intermediate in pharmaceutical synthesis, materials science, and specialized chemical research applications.
CHEMICAL INFORMATION
-
Chemical Name: 6-Bromo-1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthalene
-
CAS Number: 27452-17-1
-
Molecular Formula: C14H19Br
-
Molecular Weight: 267.21 g/mol
-
Structural Features: Substituted tetrahydronaphthalene with bromine at the 6-position and four methyl groups at the 1- and 4-positions.
The bromine substituent at the 6-position confers high electrophilicity at this site, making the compound suitable for nucleophilic substitution reactions, cross-coupling reactions (such as Suzuki, Heck, or Negishi reactions), and halogen-metal exchange protocols. The steric bulk of the tetramethyl groups enhances selectivity in reactions and can influence conformational dynamics, an important consideration in designing advanced intermediates.
PHYSICAL AND CHEMICAL PROPERTIES
6-Bromo-1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthalene | CAS 27452-17-1 is typically a colorless to pale yellow crystalline solid, with moderate solubility in common organic solvents such as dichloromethane, chloroform, and toluene. The compound exhibits excellent thermal stability under standard laboratory conditions, and the tetrahydronaphthalene core provides chemical robustness for subsequent derivatization steps.
Key chemical properties include:
-
Reactivity: Bromide functionality allows for selective halogen-based reactions.
-
Stability: Resistant to mild acids and bases; stable under ambient storage conditions.
-
Solubility: Soluble in nonpolar and slightly polar organic solvents, facilitating easy handling in synthetic protocols.
APPLICATIONS
6-Bromo-1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthalene | CAS 27452-17-1 is widely employed in research and industrial settings due to its versatile reactivity and structural features. Key applications include:
-
Pharmaceutical Intermediates:
This compound is frequently used as a building block in the synthesis of complex pharmaceuticals and bioactive molecules. Its bromine atom provides a functional handle for introducing additional substituents, enabling the preparation of molecules with precise stereochemistry and electronic properties. -
Organic Synthesis Research:
In academic and industrial research laboratories, this brominated tetrahydronaphthalene serves as a model compound for studying reaction mechanisms, halogen-metal exchange chemistry, and sterically influenced transformations. -
Material Science Applications:
The tetrahydronaphthalene scaffold and bromine substituent can be leveraged in designing specialized polymers, liquid crystals, or other advanced materials, where the compound’s structural rigidity and substituent pattern impact material properties. -
Cross-Coupling Reactions:
The bromide functionality at the 6-position is highly reactive in palladium- or nickel-catalyzed cross-coupling reactions. This enables the formation of carbon-carbon or carbon-heteroatom bonds efficiently, which is critical for synthesizing advanced organic molecules. -
Synthetic Versatility:
Due to the steric hindrance of the tetramethyl groups, this compound is often used to investigate sterically controlled reactions, selective bromination, or other chemoselective modifications in complex organic frameworks.
SAFETY AND HANDLING
While 6-Bromo-1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthalene is generally stable, standard laboratory safety protocols must be followed. It should be handled with appropriate personal protective equipment, in well-ventilated areas or under fume hoods. Avoid inhalation, ingestion, and direct skin or eye contact. Proper storage in a cool, dry environment away from incompatible reagents is recommended to maintain purity and stability.
SUMMARY
6-Bromo-1,1,4,4-tetramethyl-1,2,3,4-tetrahydronaphthalene is a high-value chemical intermediate for synthetic organic chemistry, pharmaceutical research, and materials science. Its brominated tetrahydronaphthalene core, combined with tetramethyl substitution, provides unique reactivity, steric properties, and chemical stability. As a versatile building block, it enables advanced synthetic transformations, making it an essential compound for research and industrial applications.
![4-(4-amino-1H-pyrazolo[3,4-d]pyrimidin-3-yl)phenol | CAS 1293915-57-7](https://resolvemass.ca/wp-content/uploads/2026/01/4-4-amino-1H-pyrazolo34-dpyrimidin-3-ylphenol-CAS-1293915-57-7-2.png)
Reviews
There are no reviews yet.