PRODUCT OVERVIEW
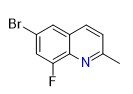
6-bromo-8-fluoro-2-methylquinoline | CAS 958650-94-7 is a highly specialized heterocyclic intermediate used predominantly in medicinal chemistry, agrochemical research, and advanced material synthesis. Characterized by a quinoline core substituted with bromine, fluorine, and methyl functionalities, this compound serves as a versatile building block for the development of novel small molecules with potential pharmacological activity.
Quinolines are a class of nitrogen-containing aromatic compounds with well-established utility in drug discovery. The strategic placement of halogen substituents — bromine at position 6 and fluorine at position 8 — along with a methyl group at position 2, imparts unique electronic, steric, and physicochemical properties that can profoundly influence biological activity, metabolic stability, and binding affinity when incorporated into target compounds.
CHEMICAL SPECIFICATIONS
| Property | Specification |
|---|---|
| Chemical Name | 6-Bromo-8-Fluoro-2-Methylquinoline |
| CAS Registry Number | 958650-94-7 |
| Molecular Formula | C10H7BrFN |
| Molecular Weight | 240.08 g/mol |
| Structure Type | Substituted quinoline |
| Physical State | Solid (typically crystalline) |
| Purity | ≥ 98.0% (by HPLC) |
| Appearance | Off-white to light yellow powder |
KEY FEATURES & ADVANTAGES
High Purity and Consistency
Manufactured under strict quality controls, 6-Bromo-8-Fluoro-2-Methylquinoline is supplied with a high degree of purity (≥98%), ensuring reliability and reproducibility in research and development workflows.
Strategic Functionalization
-
Bromine (Br) at the 6-position enables efficient cross-coupling reactions (e.g., Suzuki, Heck, Buchwald-Hartwig), a cornerstone for constructing complex heterocycles and biaryl systems.
-
Fluorine (F) at the 8-position modulates electronic density and improves metabolic resilience in drug candidates.
-
Methyl (CH<sub>3</sub>) at the 2-position provides desirable steric and lipophilic effects, influencing binding interactions.
Versatile Intermediate
This compound is an ideal substrate for derivatization, facilitating rapid exploration of structure-activity relationships (SAR) across chemical libraries.
Research and Development Focus
Valuable for synthetic chemists engaged in:
-
Lead optimization
-
Medicinal chemistry programs
-
Heterocyclic scaffolding
-
Fluorine-containing compound design
-
Agrochemical innovation
SYNTHETIC UTILITY
6-Bromo-8-Fluoro-2-Methylquinoline’s unique substitution pattern makes it a prime candidate for a range of synthetic transformations:
Cross-Coupling Reactions
The bromine substituent readily participates in palladium-catalyzed coupling reactions with organoboron, organostannane, and organozinc reagents. These reactions build complexity with high regioselectivity.
Nucleophilic Aromatic Substitution (S<sub>N</sub>Ar)
The presence of fluorine on the quinoline ring enhances opportunities for nucleophilic aromatic substitution, particularly with heteroatom nucleophiles, enabling the installation of amines, alkoxides, or thiol groups.
C–H Functionalization Approaches
Emerging catalytic methodologies allow for direct C–H activation around the quinoline core, expanding the synthetic toolbox for diversifying quinoline derivatives.
Heterocycle Expansion & Ring-Closing Strategies
This intermediate can be transformed into fused bicyclic or polycyclic frameworks, which are essential motifs in many bioactive compounds.
APPLICATIONS of 6-bromo-8-fluoro-2-methylquinoline | CAS 958650-94-7 :
Medicinal Chemistry
Quinoline derivatives have a long history of biological relevance, featuring in antimalarials, antivirals, anticancer agents, and CNS-active compounds. The incorporation of bromine and fluorine substituents can:
-
Improve binding affinity
-
Increase lipophilicity
-
Enhance metabolic stability
-
Modulate pKa and solubility
These qualities make 6-Bromo-8-Fluoro-2-Methylquinoline valuable for lead discovery and optimization campaigns.
Agrochemical Research
Halogenated quinoline scaffolds have been investigated for herbicidal, insecticidal, and fungicidal properties. This intermediate offers a pathway toward novel agrochemicals with improved efficacy and environmental profiles.
Material Science
Functionalized quinolines serve as precursors in the synthesis of organic electronic materials, dyes, ligands for coordination chemistry, and fluorophores for imaging applications.
SAR Library Development
This compound enables the construction of focused libraries for exploratory screening, particularly where halogen bonding and aromatic interactions are of interest.
ANALYTICAL CHARACTERIZATION
Each batch of 6-Bromo-8-Fluoro-2-Methylquinoline is characterized using a suite of analytical techniques to ensure identity and purity:
-
High-Performance Liquid Chromatography (HPLC) — purity assessment
-
Nuclear Magnetic Resonance (NMR) spectroscopy — structural confirmation
-
Mass Spectrometry (MS) — molecular weight verification
-
Elemental Analysis — composition validation
Certificates of Analysis (CoAs) are available with each order, documenting batch-specific results.
SAFETY & HANDLING
6-Bromo-8-Fluoro-2-Methylquinoline should be handled by trained personnel in a well-equipped laboratory environment:
-
Use appropriate personal protective equipment (gloves, goggles, lab coat).
-
Avoid inhalation, ingestion, and contact with skin or eyes.
-
Store in a cool, dry area, tightly sealed away from incompatible agents.
-
Consult the Safety Data Sheet (SDS) before use for hazard classifications, first-aid measures, and disposal guidelines.
CUSTOM SERVICES
ResolveMass Laboratories Inc. offers tailored services to support your projects, including:
-
Custom synthesis
-
Scale-up processing
-
Analytical method development
-
Purity optimization
Contact our technical support team for project consultation and bulk pricing.
Reviews
There are no reviews yet.