OVERVIEW
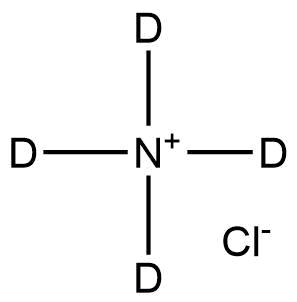
Ammonium-d₄ chloride is a deuterium-labeled inorganic compound in which all four hydrogen atoms of the ammonium ion (NH₄⁺) are replaced by deuterium, forming the ND₄⁺ cation. It is the isotopically enriched analogue of ammonium chloride (NH₄Cl) and provides a precise +4 Da mass increase, making it an essential reagent in analytical, spectroscopic, and tracer studies.
This compound is chemically stable, highly soluble in water, and exhibits identical ionic properties to its non-deuterated counterpart. Due to its deuterium enrichment, Ammonium-d₄ chloride is particularly valuable in nuclear magnetic resonance (NMR) spectroscopy, isotope tracer experiments, and studies of nitrogen metabolism and proton exchange mechanisms.
At ResolveMass Laboratories Inc., Ammonium-d₄ chloride is synthesized and purified to meet high isotopic and chemical purity standards, ensuring consistent and reproducible performance in both research and analytical applications.
CHEMICAL INFORMATION
-
Name : Ammonium-d₄ chloride
-
Molecular Formula: ND₄Cl
-
Molecular Weight: 57.51 g/mol
-
CAS Number: 12015-14-4
-
Isotopic Enrichment: ≥99 atom % D
-
Appearance: White crystalline solid
-
Stability: Stable under normal laboratory conditions; non-hygroscopic when stored properly
Ammonium-d₄ chloride’s ionic nature and complete deuterium substitution make it an ideal source of deuterated nitrogen species for isotopic exchange reactions and analytical calibration.
APPLICATIONS of Ammonium-d4 Chloride | CAS 12015-14-4
1. NMR Spectroscopy and Solvent Reference:
Ammonium-d₄ chloride is frequently used as a reference compound or solvent additive in ^2H NMR spectroscopy. It provides distinct deuterium signals, making it valuable for chemical shift calibration and solvent-locking applications in spectroscopic studies.
2. Isotopic Tracer in Chemical and Biological Systems:
Due to its high isotopic enrichment, it is used as a stable tracer in studies of nitrogen cycling, proton-deuteron exchange, and ammonia transformation. Researchers employ it to track metabolic and biochemical processes involving nitrogen and hydrogen exchange pathways.
3. Calibration Standard in Mass Spectrometry:
Its well-defined isotopic mass (+4 Da shift) makes Ammonium-d₄ chloride suitable for calibrating and validating analytical instruments such as mass spectrometers, especially in isotopic ratio and trace-level quantification experiments.
4. Deuterium Source in Synthesis:
Ammonium-d₄ chloride acts as a mild and controllable deuterium donor in reactions requiring the incorporation of deuterium into nitrogen-containing or exchangeable hydrogen positions. It is also used in preparing deuterated amines and ammonium salts.
5. Research in Hydrogen-Deuterium Exchange (HDX):
The compound is utilized in mechanistic studies involving hydrogen-deuterium exchange in both organic and inorganic systems. It provides insight into reaction kinetics, solvent effects, and catalytic isotope behavior.
6. Environmental and Isotope Ratio Analysis:
Ammonium-d₄ chloride serves as a stable isotopic marker in environmental monitoring and isotope ratio mass spectrometry (IRMS), helping scientists assess nitrogen transformations and deuterium dynamics in soil, water, and biological systems.
ADVANTAGES of Ammonium-d4 Chloride | CAS 12015-14-4
-
High Isotopic Enrichment (≥99 atom % D): Ensures precise isotopic tracing and analytical accuracy.
-
Chemically and Thermally Stable: Suitable for a wide range of laboratory and industrial uses.
-
Non-Toxic and Non-Volatile: Safer to handle compared to many deuterated organic reagents.
-
Excellent Solubility: Dissolves readily in water and polar solvents for easy use in aqueous systems.
-
Ideal for NMR and MS Calibration: Provides consistent isotopic and spectral reference points.
-
Cost-Effective Deuterium Source: Economical for synthesis and isotopic labeling applications.
HANDLING:
-
Handle in a clean, dry environment to prevent contamination.
-
Avoid inhalation of dust or direct contact with skin and eyes.
-
Use gloves, protective goggles, and laboratory clothing when handling solid material.
QUALITY & ANALYTICAL CHARACTERIZATION
ResolveMass Laboratories Inc. ensures that each batch of Ammonium-d₄ chloride undergoes rigorous analytical verification for both isotopic and chemical integrity.
Analytical Verification Includes:
-
Mass Spectrometry (MS): Confirms isotopic composition and +4 Da mass increase.
-
Infrared Spectroscopy (IR): Verifies ND₄⁺ vibration modes and Cl⁻ association.
-
NMR Spectroscopy (^2H, ^15N): Validates deuterium incorporation and structural stability.
-
Thermogravimetric Analysis (TGA): Confirms compound stability and purity.
A Certificate of Analysis (COA) and Safety Data Sheet (SDS) accompany every product, detailing isotopic enrichment, purity, and analytical results for quality assurance and traceability.
SUMMARY
Ammonium-d₄ chloride (CAS 12015-14-4) is a high-purity, fully deuterated ammonium salt designed for use in isotope labeling, NMR spectroscopy, and chemical tracing studies. Its high isotopic enrichment, thermal stability, and solubility make it a versatile reagent for research in analytical chemistry, environmental science, and biochemistry.
ResolveMass Laboratories Inc. supplies Ammonium-d₄ chloride with certified isotopic and chemical purity, supporting scientists and researchers in obtaining precise, reproducible, and reliable results in deuterium-based investigations and analytical applications.
Learn more about,
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Availability of All the Deuterated Chemicals at ResolveMass Laboratories Inc.
ResolveMass Laboratories: Leading Deuterated Chemical Synthesis Company in the United States.
Deuterated Internal Standards for LC-MS: Selection & Custom Synthesis
How to Choose the Right Deuterated Labelled Chemical Synthesis Company in Canada
How to Choose the Right Deuterium Labelled Compounds Supplier for Your Lab
Deuterium-Labelled Compounds — Synthesis, Applications & Ordering
Reviews
There are no reviews yet.