OVERVIEW
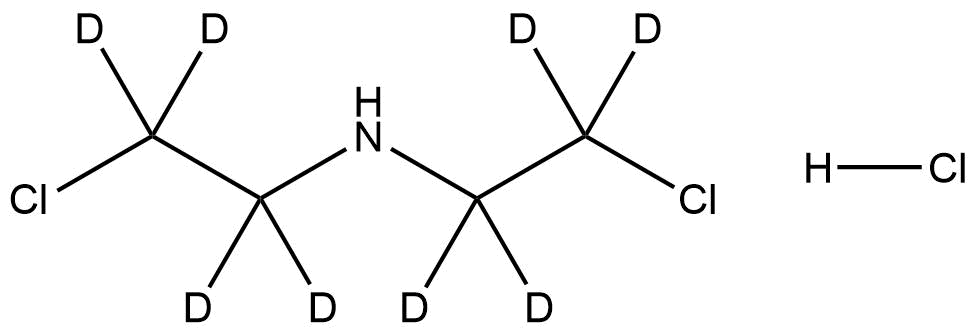
Bis(2-chloroethyl)-d₈-amine hydrochloride is a fully deuterated isotopic analogue of bis(2-chloroethyl)amine hydrochloride, featuring eight deuterium atoms substituted for hydrogen. It is a halogenated secondary amine salt with significant utility in isotope labeling, analytical chemistry, and mechanistic research. The compound’s stable isotopic substitution makes it particularly valuable for mass spectrometric and NMR investigations, allowing researchers to distinguish it from its non-deuterated counterpart with high precision.
At ResolveMass Laboratories Inc., Bis(2-chloroethyl)-d₈-amine HCl is synthesized with stringent control over isotopic enrichment and chemical purity, ensuring consistency and reproducibility across various research and industrial applications. The compound retains the core chemical reactivity of its protonated analogue while offering a higher molecular mass and distinct spectroscopic signature, making it ideal for tracing, calibration, and mechanistic studies in both organic and pharmaceutical chemistry.
CHEMICAL INFORMATION
-
Name: Bis(2-chloroethyl)-d₈-amine hydrochloride;
-
Molecular Formula: C₄D₈Cl₃N
-
Molecular Weight: 186.53 g/mol
-
CAS Number: 102092-04-6
-
Stability: Stable under recommended storage conditions; hygroscopic and light-sensitive
This compound exhibits the same chemical backbone as the non-deuterated form, with the C–D bonds providing enhanced thermal and metabolic stability. These features make it ideal for use in analytical assays requiring isotopic discrimination.
APPLICATIONS of Bis(2-chloroethyl)-d8-amine HCl | CAS 102092-04-6
1. Internal Standard for Mass Spectrometric Quantification:
Due to its +8 Da isotopic mass difference, Bis(2-chloroethyl)-d₈-amine HCl serves as a highly reliable internal standard for quantitative analysis in GC-MS and LC-MS. It enables accurate detection, calibration, and quantification of halogenated amine species, ensuring consistency in analytical results.
2. Reference Compound in NMR Spectroscopy:
The extensive deuteration minimizes proton interference, resulting in cleaner spectra in ^1H NMR and facilitating ^13C or ^15N studies. It also aids in solvent suppression experiments and kinetic isotope effect (KIE) measurements.
3. Tracer in Biochemical and Pharmacological Research:
The compound’s labeled isotopic structure allows its use in studying reaction pathways and metabolic fate of nitrogen-containing halogenated compounds. Researchers use it as a non-radioactive tracer to monitor compound transformations under controlled biological or environmental conditions.
4. Precursor for Deuterated Intermediates:
The reactive bis(2-chloroethyl) structure provides a versatile synthetic platform. It can participate in nucleophilic substitution reactions to yield a range of deuterated amine derivatives, including tertiary amines and substituted ammonium salts, useful in deuterium-labeling strategies and isotope chemistry.
5. Mechanistic and Kinetic Studies:
The C–D bonds in Bis(2-chloroethyl)-d₈-amine HCl exhibit lower vibrational frequencies than C–H bonds, which is advantageous for evaluating kinetic isotope effects in organic reactions involving deuterated substrates. This allows researchers to gain deeper insights into reaction pathways, transition-state structures, and rate-determining steps.
6. Environmental and Toxicological Simulations:
In toxicology and environmental chemistry, the compound serves as a stable isotope analog for studying the reactivity and degradation mechanisms of nitrogen mustard derivatives. The deuterium label allows precise mass tracking and monitoring of degradation intermediates.
ADVANTAGES of Bis(2-chloroethyl)-d8-amine HCl | CAS 102092-04-6
-
High Isotopic Purity (≥98 atom % D): Ensures strong isotopic differentiation and reliable analytical performance.
-
Stable and Non-Radioactive Label: Safer alternative to radiolabeling methods for tracing and quantification.
-
Enhanced Mass Resolution: Distinct isotopic signature allows easy identification in MS-based systems.
-
Comparable Chemical Reactivity: Maintains the same reaction behavior as the unlabeled compound.
-
Improved Thermal and Chemical Stability: Due to stronger C–D bonds compared to C–H.
-
Versatile Research Utility: Suitable for use in analytical, mechanistic, and synthetic studies.
HANDLING
-
Handle in a well-ventilated fume hood to minimize inhalation exposure.
-
Avoid direct contact with skin and eyes; use appropriate protective clothing, gloves, and safety goggles.
-
Prevent exposure to heat, light, and moisture to maintain compound integrity.
-
Follow standard laboratory protocols for handling chlorinated amines.
QUALITY & ANALYTICAL CHARACTERIZATION
ResolveMass Laboratories Inc. ensures high isotopic and chemical purity through rigorous analytical testing of each production batch.
Analytical Verification Includes:
-
Mass Spectrometry (MS): Confirms molecular ion peak and isotopic distribution.
-
NMR Spectroscopy (^1H, ^2H, ^13C, ^15N): Determines isotopic substitution levels and structural integrity.
-
Infrared Spectroscopy (IR): Detects characteristic C–D stretching vibrations.
-
Elemental Analysis: Confirms empirical composition and purity.
Each batch is accompanied by a detailed Certificate of Analysis (COA) and Safety Data Sheet (SDS) to ensure traceability, reproducibility, and compliance with research and regulatory standards.
SUMMARY
Bis(2-chloroethyl)-d₈-amine hydrochloride (CAS 102092-04-6) is a premium-quality deuterated compound designed for use in isotope labeling, analytical calibration, and mechanistic research. With high isotopic enrichment and exceptional purity, it provides superior reliability in GC-MS, LC-MS, and NMR applications.
Its combination of chemical reactivity, isotopic precision, and structural stability makes it a key resource for scientists engaged in deuterium-labeling, environmental tracing, and kinetic isotope effect studies. ResolveMass Laboratories Inc. delivers this compound under strict quality control standards, supporting cutting-edge analytical and synthetic research worldwide.
Learn more about,
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Availability of All the Deuterated Chemicals at ResolveMass Laboratories Inc.
ResolveMass Laboratories: Leading Deuterated Chemical Synthesis Company in the United States.
Deuterated Internal Standards for LC-MS: Selection & Custom Synthesis
How to Choose the Right Deuterated Labelled Chemical Synthesis Company in Canada
How to Choose the Right Deuterium Labelled Compounds Supplier for Your Lab
Deuterium-Labelled Compounds — Synthesis, Applications & Ordering
Reviews
There are no reviews yet.