OVERVIEW
Deuterated Methyl-d3 methacrylate | CAS 35777-12-9 is an isotopically labeled analogue of methyl methacrylate in which the methoxy group contains three deuterium atoms instead of hydrogen. This strategic substitution retains the chemical and polymerization behavior of the parent monomer while significantly enhancing contrast in nuclear-based analytical techniques. Because deuterium interacts differently with neutrons and has unique NMR signatures, this labeled monomer provides a powerful way to track polymer segments, analyze morphology, and study molecular dynamics in systems where conventional characterization techniques fall short.
CHEMICAL INFORMATION
-
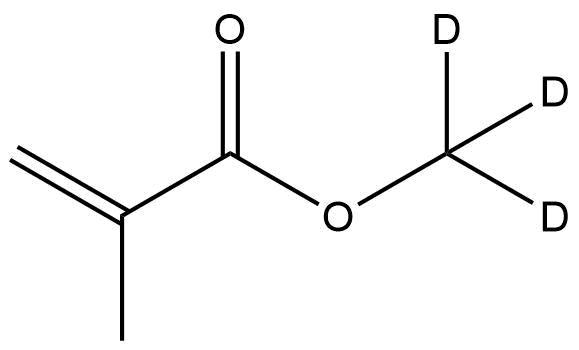
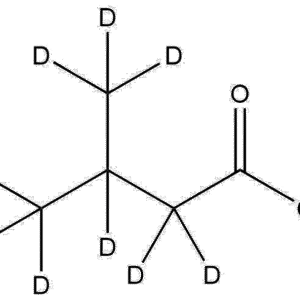
Chemical Name: Deuterated Methyl-d₃ Methacrylate
-
Synonyms: Methyl Methacrylate-d₃, Methacrylic Acid Methyl-d₃ Ester
-
CAS Number: 35777-12-9
-
Molecular Formula: C₅H₅D₃O₂
-
Molecular Weight: ~103.13 g/mol
-
Structure: Deuterium substitution occurs specifically at the methyl group attached to the oxygen atom, providing isotopic labeling without changing the core methacrylate functionality.
This compound behaves almost identically to standard MMA during free-radical polymerization, controlled radical polymerization (RAFT/ATRP), and copolymerization with acrylates, methacrylates, and functional monomers. Its high isotopic purity enhances analytical resolution, making it ideal for advanced structural investigations.
KEY FEATURES
1. Enhanced Neutron Scattering Contrast
The difference in neutron scattering cross-sections between hydrogen and deuterium enables precise structural mapping in thin films, copolymers, blends, and nanoscale materials. Incorporating this monomer into specific segments of a polymer chain provides tunable contrast for small-angle neutron scattering (SANS), neutron reflectometry, and related techniques.
2. Superior ²H NMR Tracking Capability
Introducing deuterium atoms into defined molecular positions allows clean, interference-free ²H NMR detection. This enables researchers to probe segmental mobility, domain relaxation, chain orientation, and local microenvironments within bulk polymers, gels, and hybrid materials.
3. Chemically Equivalent to Native Methyl Methacrylate
Because deuterium substitution does not significantly alter steric or electronic properties, polymerization kinetics, mechanical behavior, and thermal performance remain closely aligned with standard PMMA systems. This ensures highly meaningful comparisons in mechanistic studies or when blending labeled and unlabeled materials.
4. Useful in Mechanistic & Kinetic Polymerization Studies
Isotopically labeled monomers are valuable for exploring chain transfer pathways, sequence distribution, and incorporation patterns. Deuterium labeling offers a clean, quantifiable way to distinguish between different monomer units in complex copolymer systems.
APPLICATIONS of Deuterated Methyl-d3 methacrylate | CAS 35777-12-9
• Deuterated PMMA & PMMA Copolymers
Used to prepare isotopically labeled polymers for neutron reflectometry, SANS, and advanced morphological analysis. Researchers often substitute a controlled fraction of the MMA feed with its d₃ analogue to create a contrast-selective polymer segment.
• Block Copolymer Nanostructures
Ideal for studies involving domain spacing, interfacial behavior, and self-assembly in block copolymers where one block is deuterated to selectively highlight specific regions.
• Polymer Dynamics & Relaxation Studies
The defined placement of deuterium atoms enables precise tracking of chain motion in solid-state and solution-state systems using ²H NMR methods.
• Soft-Matter, Colloid, and Interface Science
Used to study polymer brushes, coatings, surface modifications, diffusion processes, and interfacial phenomena that require isotopic distinction without altering chemical properties.
• Analytical Method Development
Functions as an internal standard or reference material in workflows designed around isotopic labeling, enabling accurate quantification and structural elucidation.
HANDLING & SAFETY NOTES
Deuterated Methyl-d₃ Methacrylate should be handled with the same caution required for all methacrylate monomers. It may undergo unwanted polymerization if exposed to heat, light, or radical initiators. Store under inert conditions, use appropriate personal protective equipment, and ensure good ventilation. Consult the SDS for detailed safety and handling procedures.
WHY CHOOSE RESOLVEMASS LABORATORIES
ResolveMass Laboratories Inc. provides high-purity, isotopically enriched monomers with reliable quality documentation and analytical verification. Our materials support advanced R&D across polymer chemistry, neutron scattering, NMR studies, and precision materials engineering. With a focus on reproducibility, consistency, and scientific integrity, we ensure researchers can confidently incorporate deuterated monomers into their experimental workflows.
Learn more through,
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Availability of All the Deuterated Chemicals at ResolveMass Laboratories Inc.
ResolveMass Laboratories: Leading Deuterated Chemical Synthesis Company in the United States.
Deuterated Internal Standards for LC-MS: Selection & Custom Synthesis
How to Choose the Right Deuterated Labelled Chemical Synthesis Company in Canada
How to Choose the Right Deuterium Labelled Compounds Supplier for Your Lab
Deuterium-Labelled Compounds — Synthesis, Applications & Ordering
Custom Synthesis of Deuterated Chemicals
Reviews
There are no reviews yet.