OVERVIEW of Ethylene-d4-diamine 2 HCl | CAS 34334-71-9
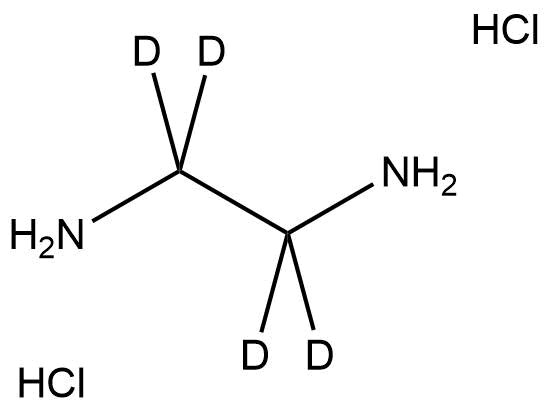
Ethylene-d₄-diamine·2HCl (CAS 34334-71-9) is a fully deuterated form of ethylenediamine, supplied as its dihydrochloride salt for greater chemical stability and ease of handling. In this isotopologue, all four hydrogen atoms on the carbon backbone are replaced by deuterium, giving the compound unique utility as a stable isotope-labeled reagent. It is extensively used in NMR spectroscopy, mass spectrometry, isotopic tracing, and mechanistic chemistry studies, where it functions as a key analytical reference or labeled intermediate.
CHEMICAL INFORMATION
-
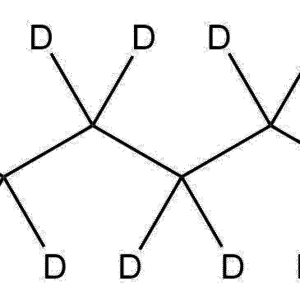
Name: Ethylenediamine-d₄ dihydrochloride
-
Molecular Formula: C₂D₄N₂·2HCl
-
Molecular Weight: 137.04 g/mol
-
CAS Number: 34334-71-9
-
Isotopic Enrichment: ≥ 98 atom % D
-
Chemical Class: Deuterated diamine salt
-
Solubility: Freely soluble in water and alcohols
APPLICATIONS of Ethylene-d4-diamine 2 HCl | CAS 34334-71-9
-
NMR Spectroscopy:
Ethylene-d₄-diamine·2HCl is a preferred deuterium-labeled internal reference for structural and kinetic NMR studies. The substitution of deuterium eliminates proton coupling, thereby enhancing spectral clarity and enabling accurate identification of nitrogen-bound chemical environments.
-
Mass Spectrometry (MS):
Widely used as an isotopically distinct standard in MS calibration and quantitation of amine-containing compounds. The deuterated variant provides clear mass separation, improving analytical precision and accuracy.
-
Isotopic Tracer Studies:
Acts as a deuterium tracer in biochemical and chemical kinetic studies. Researchers employ it to follow hydrogen–deuterium exchange, reaction intermediates, or degradation pathways of diamine derivatives.
-
Synthetic Chemistry:
Functions as a labeled precursor in the synthesis of deuterium-enriched complexes, chelating agents, and pharmaceutical intermediates. The dihydrochloride salt ensures greater control and reproducibility in reactions compared to the free base.
ADVANTAGES of Ethylene-d4-diamine 2 HCl | CAS 34334-71-9
-
High isotopic enrichment (≥ 98 atom % D) ensures reliable isotope labeling.
-
Stable crystalline form minimizes degradation or volatility.
-
Enhanced solubility in aqueous and polar media.
-
Ideal for both analytical and preparative deuteration studies.
-
Compatible with a wide range of spectroscopic and synthetic methods.
HANDLING AND SAFETY
-
Handling Precautions: Avoid inhalation and direct contact; handle under dry, inert conditions.
-
Protective Equipment: Laboratory gloves, safety goggles, and suitable protective clothing are recommended.
-
Storage: Keep container tightly sealed in a cool, dry environment, away from acids and oxidizing agents.
-
Stability: Stable under normal laboratory conditions; hygroscopic when exposed to air.
-
Disposal: Dispose of according to institutional and regional guidelines for amine hydrochlorides and deuterated chemicals.
SUMMARY
Ethylene-d₄-diamine·2HCl (CAS 34334-71-9) is a fully deuterated ethylenediamine dihydrochloride designed for high-precision analytical, isotopic, and synthetic applications. Its superior isotopic purity, chemical stability, and solubility make it a valuable standard for NMR and MS analyses, as well as a reliable reagent for mechanistic and kinetic studies in both chemical and biochemical research.
Learn more about,
Deuterated Polymers: A Cornerstone Guide to Synthesis, Applications, and Future Trends
Availability of All the Deuterated Chemicals at ResolveMass Laboratories Inc.
ResolveMass Laboratories: Leading Deuterated Chemical Synthesis Company in the United States.
Deuterated Internal Standards for LC-MS: Selection & Custom Synthesis
How to Choose the Right Deuterated Labelled Chemical Synthesis Company in Canada
How to Choose the Right Deuterium Labelled Compounds Supplier for Your Lab
Deuterium-Labelled Compounds — Synthesis, Applications & Ordering
Custom Synthesis of Deuterated Chemicals
PubChem CID
Reviews
There are no reviews yet.