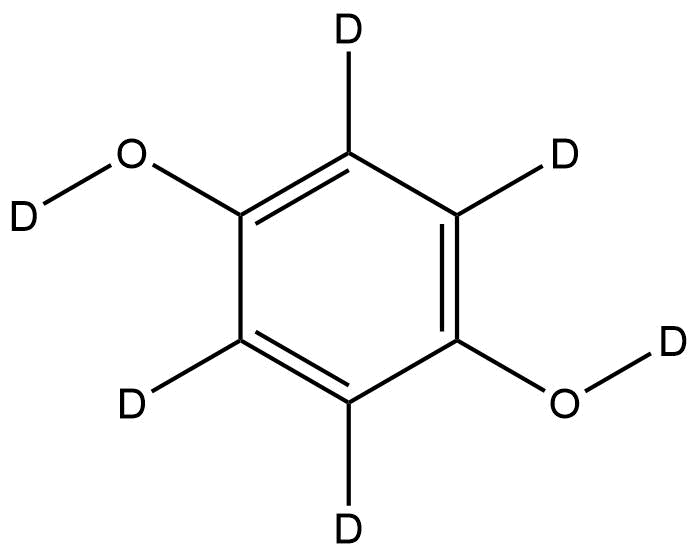
Hydroquinone-d6 | CAS No.: 71589-26-9
Chemical Name: Hydroquinone-d6
Synonyms: p-Dihydroxybenzene-d6, 1,4-Benzenediol-d6, p-Benzenediol-d6
Molecular Formula: C₆D₆O₂
Molecular Weight: 116.15 g/mol
Isotopic Purity: ≥ 98 atom % D
Chemical Structure:
(Deuterated analog of hydroquinone; aromatic ring fully deuterated at all six hydrogen positions)
Product Description
Hydroquinone-d6 is a fully deuterated isotopologue of hydroquinone, in which all six aromatic hydrogens are replaced by deuterium atoms. This compound retains the same phenolic structure as hydroquinone (1,4-dihydroxybenzene) but with enhanced mass stability and isotopic labeling that makes it invaluable for advanced analytical and mechanistic studies.
Hydroquinone-d6 is primarily used as an internal standard and reference compound in mass spectrometry (MS) and nuclear magnetic resonance (NMR) analyses. Due to its chemical similarity to native hydroquinone and isotopic differentiation (mass +6), it provides accurate quantitation and tracing in complex analytical systems without interfering with the analyte of interest.
Applications
1. Internal Standard in LC-MS and GC-MS
Hydroquinone-d6 serves as a stable isotopic internal standard for the quantitative analysis of hydroquinone and related phenolic compounds. In liquid chromatography–mass spectrometry (LC-MS) or gas chromatography–mass spectrometry (GC-MS) workflows, the deuterated analog allows precise quantitation by correcting for sample losses, matrix effects, and ion suppression phenomena.
Researchers employ Hydroquinone-d6 in:
-
Environmental monitoring of phenolic pollutants.
-
Pharmaceutical impurity profiling where hydroquinone appears as a degradation product.
-
Cosmetic product testing, where hydroquinone derivatives are analyzed for stability and safety.
2. Mechanistic Studies in Redox Chemistry
Hydroquinone participates in reversible redox transformations to p-benzoquinone, serving as a model system for studying electron transfer and oxidative stress processes. The deuterated form (Hydroquinone-d6) provides valuable insight into kinetic isotope effects (KIE) during oxidation reactions, enabling researchers to evaluate reaction pathways, rate-determining steps, and hydrogen (deuterium) transfer mechanisms.
3. NMR and Spectroscopic Research
Hydroquinone-d6 is used in NMR spectroscopy as a reference material and solvent compatibility control. The deuterated ring eliminates proton signals from the aromatic positions, simplifying spectra and enhancing signal clarity. It’s also useful for calibrating chemical shift referencing or studying exchange phenomena between hydroxyl protons and solvent systems.
4. Metabolic and Toxicological Research
In biochemical studies, Hydroquinone-d6 is applied as a tracer molecule to study hydroquinone metabolism, particularly in toxicological assessments of benzene exposure and oxidative damage. Its isotopic label allows the distinction between endogenous and exogenous sources of hydroquinone in biological samples.
Synthesis and Quality
Hydroquinone-d6 is synthesized via deuterium exchange or catalytic aromatic deuteration using high-purity deuterium gas or deuterated solvents. The synthesis is conducted under strictly controlled conditions to achieve uniform isotopic labeling across all six ring positions without compromising phenolic functionality.
At ResolveMass Laboratories Inc., each batch of Hydroquinone-d6 undergoes rigorous analytical characterization, including:
-
¹H and ²H NMR spectroscopy to confirm deuteration extent.
-
GC-MS / LC-MS to verify isotopic purity and molecular weight.
-
HPLC for chemical purity assessment (>98%).
All products are supplied with a certificate of analysis (CoA) detailing isotopic enrichment, purity, and storage guidelines.
Storage and Handling
Hydroquinone-d6 should be stored in tightly closed containers under inert atmosphere conditions, ideally under nitrogen or argon. Exposure to light, oxygen, or high humidity can lead to gradual oxidation to benzoquinone. For long-term stability, it is recommended to store below 25°C and minimize repeated freeze-thaw cycles.
As with all phenolic compounds, handle Hydroquinone-d6 with appropriate personal protective equipment (PPE), including gloves, goggles, and lab coats. Avoid direct contact and inhalation of dust.
Key Advantages
-
High isotopic purity (≥98% D) for reliable quantitative studies
-
Excellent stability under normal laboratory conditions
-
Compatible with LC-MS, GC-MS, and NMR platforms
-
Useful in environmental, pharmaceutical, and biochemical research
-
Supplied with full analytical documentation for traceability
For bulk requirements, custom packaging, or isotopic customization, please contact ResolveMass Laboratories Inc. at info@resolvemass.ca.
Reviews
There are no reviews yet.