PRODUCT OVERVIEW
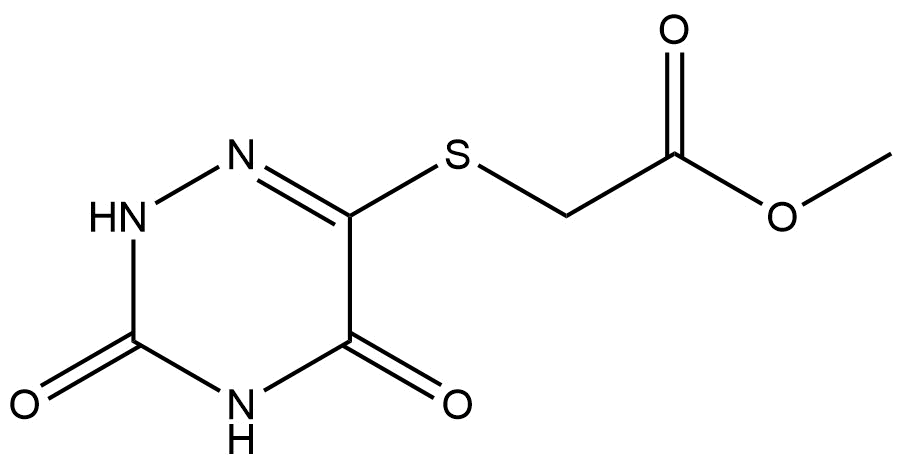
methyl 2-((3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazin-6-yl)thio)acetate | CAS 75621-68-0 is a structurally complex heterocyclic compound designed for advanced research and development applications. This molecule integrates a sulfur-linked acetate ester with a 1,2,4-triazinone core, resulting in a versatile intermediate that supports a wide range of synthetic and medicinal chemistry workflows. Due to its multifunctional architecture and chemical stability, it is frequently utilized in discovery-stage research, method development, and scaffold optimization studies.
At ResolveMass Laboratories Inc., this compound is supplied to support high-precision research where reproducibility, structural integrity, and analytical reliability are critical.
CHEMICAL IDENTITY & STRUCTURE
methyl 2-((3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazin-6-yl)thio)acetate | CAS 75621-68-0 is defined by a partially saturated 1,2,4-triazin-6-yl heterocycle substituted with two carbonyl groups at the 3- and 5-positions. The sulfur atom at the 6-position forms a thioether linkage to a methyl acetate moiety, creating a hybrid structure that combines heterocyclic nitrogen chemistry with sulfur-based functionality and ester reactivity.
-
CAS Number: 75621-68-0
-
Molecular Formula: C₆H₇N₃O₄S
-
Molecular Weight: 217.20 g/mol
The coexistence of nitrogen, oxygen, and sulfur heteroatoms contributes to the compound’s polarity, hydrogen-bonding capability, and chemical versatility, making it a valuable intermediate for downstream molecular modification.
PHYSICAL & CHEMICAL PROPERTIES
This compound is typically obtained as a solid under standard laboratory conditions. Its heterocyclic framework and ester functionality influence both solubility and reactivity:
-
Exhibits moderate polarity due to multiple heteroatoms
-
Demonstrates enhanced solubility in polar organic solvents such as DMSO and DMF
-
Limited aqueous solubility, which may increase under specific pH conditions
-
Chemically stable under neutral conditions
The ester group provides a convenient handle for hydrolysis, amidation, or coupling reactions, while the thioether linkage offers opportunities for oxidation or substitution chemistry.
SYNTHETIC & CHEMICAL FUNCTIONALITY
Methyl 2-((3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazin-6-yl)thio)acetate is primarily valued as a synthetic intermediate rather than a final active compound. Its molecular design enables chemists to explore multiple transformation pathways:
-
Ester Modifications: Conversion to carboxylic acids, amides, or substituted esters
-
Sulfur Chemistry: Oxidation to sulfoxides or sulfones, or substitution reactions
-
Heterocyclic Expansion: Functionalization of the triazinone core for analog development
These features make it particularly useful in medicinal chemistry programs focused on heterocyclic scaffolds, where small structural changes can significantly influence biological performance.
APPLICATIONS IN RESEARCH & DEVELOPMENT
This compound finds application across a broad range of scientific disciplines:
-
Medicinal Chemistry: Used as a building block for lead generation and structure–activity relationship (SAR) studies
-
Synthetic Chemistry: Serves as an intermediate for multi-step reaction sequences involving sulfur and nitrogen heterocycles
-
Method Development: Useful for validating reaction conditions, purification strategies, and analytical methods
-
Chemical Biology: Incorporated into probe molecules or modified scaffolds for biochemical evaluation
Its adaptability makes it suitable for both exploratory research and targeted synthetic programs.
ANALYTICAL CHARACTERIZATION
For research-grade use, identity and purity of methyl 2-((3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazin-6-yl)thio)acetate are typically confirmed using advanced analytical techniques:
-
NMR Spectroscopy: Structural confirmation and impurity profiling
-
Mass Spectrometry: Accurate mass verification and fragmentation analysis
-
Chromatographic Techniques: Purity assessment and stability monitoring
ResolveMass Laboratories Inc. emphasizes robust analytical characterization to ensure consistency and reliability across research applications.
SAFETY & HANDLING INFORMATION
This compound is intended strictly for research use and should be handled by trained professionals in a laboratory environment:
-
Use appropriate personal protective equipment, including gloves and eye protection
-
Avoid inhalation, ingestion, and prolonged skin contact
-
Store in a cool, dry, and well-ventilated area, protected from moisture and incompatible reagents
-
Dispose of material according to applicable laboratory and regulatory guidelines
Refer to the Safety Data Sheet (SDS) for detailed handling and safety information.
RESEARCH USE STATEMENT
Methyl 2-((3,5-dioxo-2,3,4,5-tetrahydro-1,2,4-triazin-6-yl)thio)acetate is supplied for laboratory research and development purposes only. It is not intended for diagnostic, therapeutic, or commercial use.
![5-bromo-6-iodo-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine | CAS 2549186-23-2](https://resolvemass.ca/wp-content/uploads/2026/01/5-bromo-6-iodo-7-methyl-7H-pyrrolo23-dpyrimidin-4-amineCAS-2549186-23-2-300x300.png)
![2-(3-methyl-6-nitro-3H-imidazo[4,5-b]pyridin-2-yl)ethan-1-amine | CAS 1784267-01-1](https://resolvemass.ca/wp-content/uploads/2026/01/2-3-methyl-6-nitro-3H-imidazo45-bpyridin-2-ylethan-1-amine-CAS-1784267-01-1-300x300.png)
Reviews
There are no reviews yet.