PRODUCT NAME
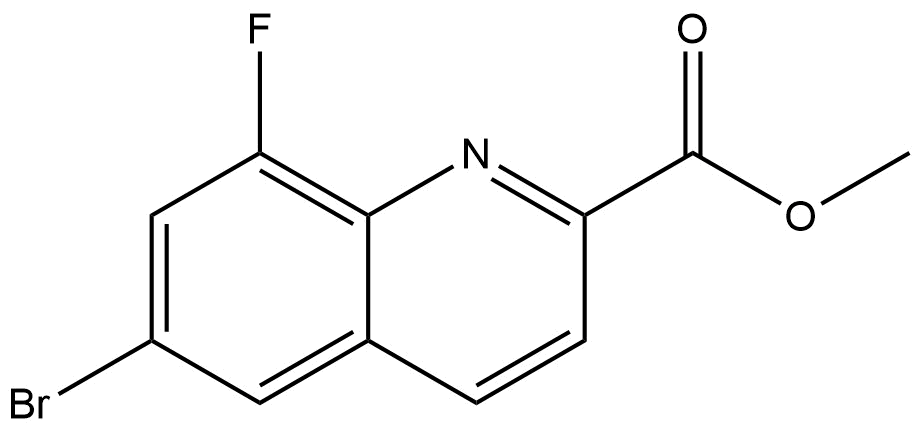
Methyl 6-Bromo-8-Fluoroquinoline-2-Carboxylate
-
CAS Number: 595748-33-6
-
Chemical Class: Halogenated quinoline ester
-
Intended Use: Research and development only
methyl 6-bromo-8-fluoroquinoline-2-carboxylate | CAS 595748-33-6 is a structurally complex halogen-substituted quinoline derivative designed for use in advanced chemical research. Featuring a quinoline heterocycle substituted with both bromine and fluorine atoms, along with a methyl ester functionality, this compound serves as a versatile intermediate in medicinal chemistry, synthetic organic chemistry, and related R&D applications.
CHEMICAL OVERVIEW
This compound is based on the quinoline core, a bicyclic aromatic heterocycle widely recognized for its importance in pharmaceutical and functional molecule design. The 2-carboxylate ester group introduces synthetic flexibility, while the 6-bromo and 8-fluoro substituents modulate both chemical reactivity and physicochemical behavior.
Halogen substitution plays a critical role in modern chemical design. The bromine atom provides a reactive site for metal-catalyzed cross-coupling reactions, while the fluorine atom contributes to enhanced molecular stability, lipophilicity control, and electronic modulation. Together, these features make methyl 6-bromo-8-fluoroquinoline-2-carboxylate a valuable scaffold for downstream chemical transformations.
MOLECULAR & STRUCTURAL CHARACTERISTICS
-
Molecular Formula: C₁₁H₇BrFNO₂
-
Core Structure: Quinoline heterocycle
-
Key Functional Groups:
-
Methyl ester (–COOCH₃)
-
Aromatic bromine substituent
-
Aromatic fluorine substituent
-
The quinoline nucleus enables conjugation and aromatic stability, making it suitable for applications requiring robust chemical frameworks. The ester functionality at the 2-position allows for further derivatization, including hydrolysis to the corresponding acid or conversion into amides and other functional groups.
PHYSICAL & CHEMICAL PROPERTIES
methyl 6-bromo-8-fluoroquinoline-2-carboxylate | CAS 595748-33-6 is typically encountered as a solid or crystalline material suitable for laboratory handling. Like other halogenated quinoline esters, it exhibits good compatibility with standard organic solvents commonly used in synthetic and analytical workflows.
Key general characteristics include:
-
Chemical stability under normal laboratory conditions
-
Solubility in a range of aprotic organic solvents
-
Aromatic reactivity suitable for catalytic transformations
The compound is designed to perform reliably as a synthetic intermediate in multi-step reaction sequences.
SYNTHETIC VALUE & REACTIVITY
This molecule is particularly valuable as a synthetic building block. The bromine substituent enables a wide range of palladium-catalyzed cross-coupling reactions, including Suzuki, Heck, and related methodologies. These reactions allow researchers to introduce diverse substituents onto the quinoline ring while preserving the fluorine and ester functionalities.
The methyl ester group further expands synthetic options by enabling:
-
Ester hydrolysis to access the corresponding carboxylic acid
-
Conversion into amides, hydrazides, or other derivatives
-
Use as a directing or protecting group during multi-step synthesis
Such versatility makes this compound well suited for library synthesis and lead optimization programs.
APPLICATIONS of methyl 6-bromo-8-fluoroquinoline-2-carboxylate | CAS 595748-33-6
Medicinal Chemistry
Quinoline derivatives are widely investigated in drug discovery due to their presence in numerous therapeutic classes. Methyl 6-bromo-8-fluoroquinoline-2-carboxylate can serve as a key intermediate in the development of bioactive molecules, supporting structure–activity relationship (SAR) studies and lead expansion strategies.
Pharmaceutical & Fine Chemical Intermediates
The compound is useful in the synthesis of more complex heterocycles and functional molecules, especially where precise substitution patterns are required.
Synthetic Method Development
Researchers use halogenated quinoline esters as test substrates for developing and optimizing new catalytic, coupling, and functionalization methods.
Materials & Functional Molecules
Quinoline-based compounds are also explored in materials science, including coordination chemistry, ligand development, and optoelectronic research, where conjugated heterocycles play a critical role.
QUALITY & TECHNICAL SUPPORT
At ResolveMass Laboratories Inc., we are committed to supplying high-quality research chemicals that meet the expectations of academic, pharmaceutical, and industrial scientists. Our products are supported by rigorous internal quality control processes and are supplied with appropriate technical documentation upon request.
Our expertise in analytical and synthetic chemistry ensures consistent product performance, making ResolveMass a trusted partner for advanced chemical research.
![4-(4-amino-1H-pyrazolo[3,4-d]pyrimidin-3-yl)phenol | CAS 1293915-57-7](https://resolvemass.ca/wp-content/uploads/2026/01/4-4-amino-1H-pyrazolo34-dpyrimidin-3-ylphenol-CAS-1293915-57-7-2.png)
![5-bromo-6-iodo-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine | CAS 2549186-23-2](https://resolvemass.ca/wp-content/uploads/2026/01/5-bromo-6-iodo-7-methyl-7H-pyrrolo23-dpyrimidin-4-amineCAS-2549186-23-2-300x300.png)
Reviews
There are no reviews yet.