PRODUCT OVERVIEW
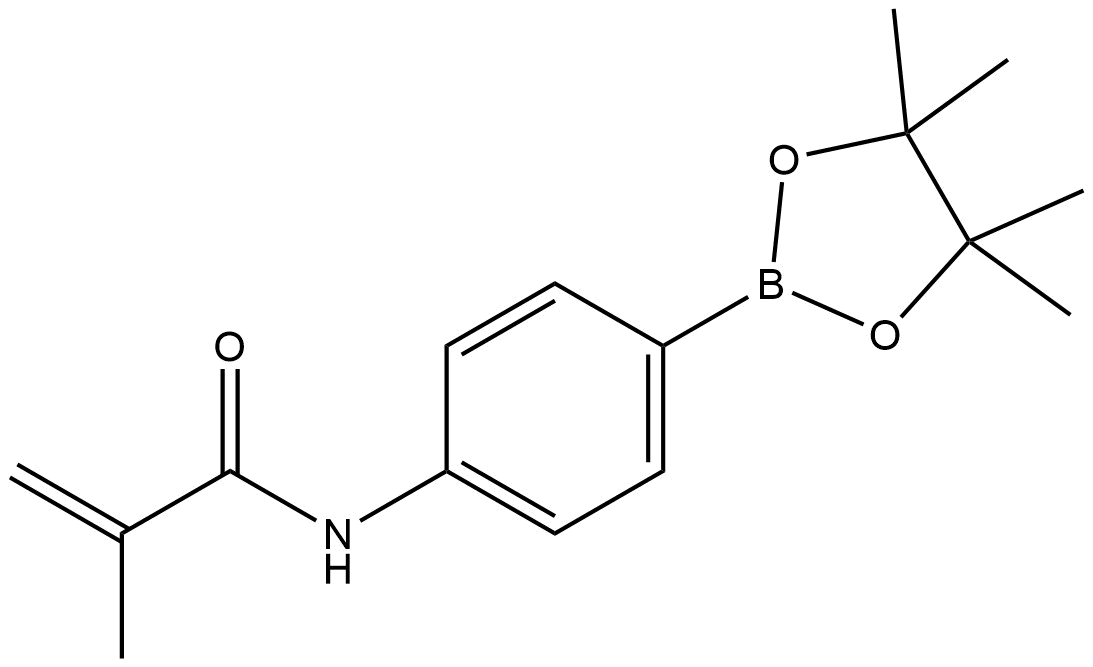
N-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)methacrylamide | CAS 1056904-41-6 is a specialized boronate ester derivative of methacrylamide, featuring a pinacol boronate functional group tethered to a para-substituted phenyl ring. This compound is prized in modern organic synthesis, medicinal chemistry, and materials science due to its versatile reactivity in cross-coupling reactions, particularly Suzuki–Miyaura couplings, and as a building block for functional polymers and complex molecular architectures.
CHEMICAL IDENTITY
-
IUPAC Name: N-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)methacrylamide
-
CAS Registry Number: 1056904-41-6
-
Molecular Formula: C16H22BNO3
-
Molecular Weight: 281.17 g/mol
PHYSICAL & CHEMICAL PROPERTIES
This compound is generally a white to off-white solid with physicochemical properties optimized for synthetic utility:
-
Appearance: Crystalline solid
-
Solubility: Soluble in common organic solvents (e.g., dichloromethane, ethyl acetate, THF); limited solubility in water
-
Reactivity Profile: The boronate ester group is stable under typical conditions but actively participates in palladium-catalyzed cross-couplings, while the methacrylamide group is amenable to radical and ionic polymerization processes.
Note: Specific melting point and spectral data (NMR, MS, IR) are provided with each lot for quality assurance.
SYNTHETIC UTILITY & REACTIVITY
Boronic Ester Cross-Coupling
The pinacol boronate ester in N-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)methacrylamide is a renowned partner in Suzuki–Miyaura cross-coupling reactions. These reactions allow for the formation of robust C–C bonds with a wide array of aryl and vinyl halides under palladium catalysis, enabling the synthesis of biaryl, styrene, and heteroaromatic derivatives.
Because boronate esters are more stable to air and moisture than free boronic acids, this compound delivers:
-
High coupling efficiencies
-
Reduced protodeboronation
-
Broad functional group tolerance
This makes it an excellent intermediate in medicinal chemistry lead optimization and complex organic molecule construction.
Polymer Synthesis Potential
The methacrylamide group enables this compound to serve as a comonomer in radical polymerizations. When incorporated into copolymer chains, the boronate function provides a reactive handle for post-polymerization modifications or cross-linking strategies. Polymers derived from this monomer can feature:
-
Tunable mechanical properties
-
Responsive or stimuli-sensitive behaviour (e.g., boronate affinity to diols)
-
Targeted functionalization via Suzuki coupling on polymer backbones
This dual functionality makes it highly attractive in advanced materials research, including smart hydrogels, sensing platforms, and stimuli-responsive coatings.
APPLICATION AREAS of N-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)methacrylamide | CAS 1056904-41-6
Medicinal & Pharmaceutical Chemistry
-
Lead diversification: Efficient coupling with heteroaryl halides expands chemical space.
-
Bioactive scaffolds: Enables synthesis of phenyl-vinylbackbone motifs common in drug candidates.
-
Fragment-based design: The boronate group can act as a masked boronic acid for enzyme-targeted programs.
Materials Science & Functional Polymers
-
Functional copolymers: Integration into polymer chains opens avenues for chemical post-modification.
-
Sensor development: Boronate affinity to cis-diols supports glucose-responsive materials and diagnostic sensors.
-
Smart materials: Incorporation in dynamic cross-linked networks for self-healing or adaptive systems.
Organic Synthesis & Methodology Development
-
Cross-Coupling Methodologies: Ideal substrate for exploring new catalytic systems and ligands.
-
Library Synthesis: Enables rapid generation of compound libraries for screening.
QUALITY & ANALYTICAL CHARACTERIZATION
ResolveMass Laboratories Inc. supplies this compound with stringent analytical documentation to ensure reproducibility in research and development. Typical quality assurance data include:
-
^1H and ^13C NMR Spectra: Confirm structural integrity
-
Mass Spectrometry (MS): Validates molecular weight
-
HPLC/GC Purity: Typically ≥ 98% (lot-specific certificate provided)
-
Elemental Analysis: Demonstrates batch consistency
Our quality control adheres to rigorous internal standards to support advanced applications across industries.
SAFETY INFORMATION
This compound should be treated as a chemical reagent with potential health and environmental hazards. Safety practices include:
-
Avoid inhalation, ingestion, and skin contact.
-
Work in a well-ventilated fume hood.
-
Refer to the Safety Data Sheet (SDS) provided by ResolveMass Laboratories for comprehensive hazards, first aid measures, and disposal guidelines.
Standard laboratory chemical hygiene practices are strongly advised.
![6-bromo-5-iodo-7-methyl-7H-pyrrolo[2,3-d]pyrimidin-4-amine | CAS 2894018-46-1](https://resolvemass.ca/wp-content/uploads/2026/01/6-bromo-5-iodo-7-methyl-7H-pyrrolo23-dpyrimidin-4-amineCAS-2894018-46-1-300x300.png)
![3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine | CAS 151266-23-8](https://resolvemass.ca/wp-content/uploads/2026/01/3-iodo-1H-pyrazolo34-dpyrimidin-4-amineCAS-151266-23-8-300x300.png)
Reviews
There are no reviews yet.