OVERVIEW
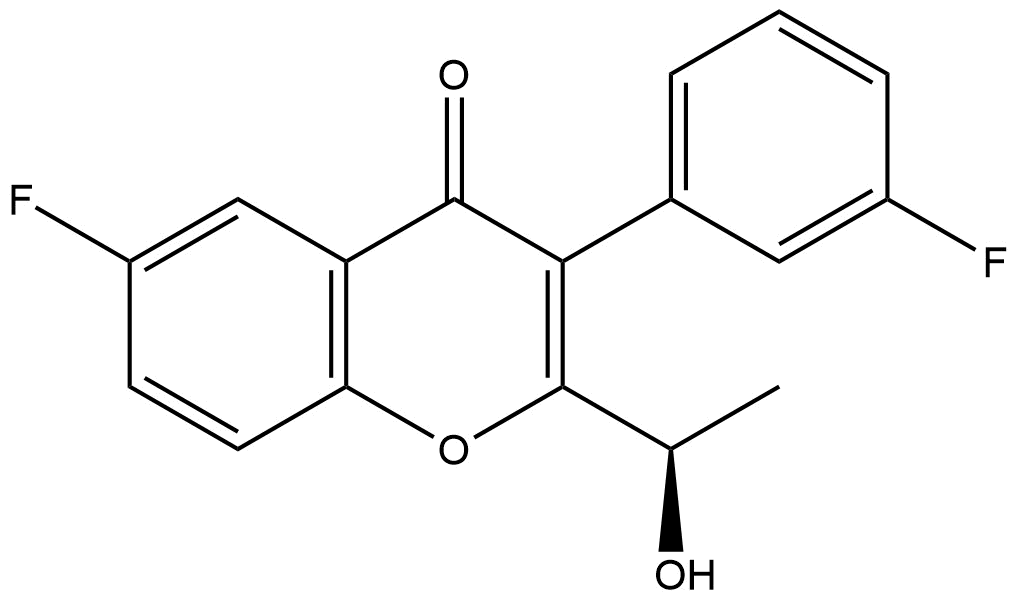
(R)-6-fluoro-3-(3-fluorophenyl)-2-(1-hydroxyethyl)-4H-chromen-4-one | CAS 1479107-10-2 is a high-purity, chiral flavanone derivative of significant utility in medicinal chemistry research and drug discovery programs. Its distinctive stereochemistry and fluorinated aromatic structure make it a valuable intermediate or test compound in the development of bioactive small molecules, particularly within therapeutic areas focusing on enzyme modulation, receptor binding optimization, and fluorine-enabled molecular tuning.
This compound’s unique combination of a chiral alcohol functional group, two fluorine substituents, and a chromenone core supports SAR (Structure-Activity Relationship) studies and advanced synthesis strategies for pharmaceutical research and specialty chemical applications. Its enantiomeric form (R-configuration) ensures predictable stereospecific interactions in chiral environments, critical for lead optimization and mechanistic investigations.
CHEMICAL INFORMATION
-
Name: (R)-6-fluoro-3-(3-fluorophenyl)-2-(1-hydroxyethyl)-4H-chromen-4-one
-
CAS Number: 1479107-10-2
-
Molecular Formula: C₁₇H₁₂F₂O₃
-
Molecular Weight: 302.28 g/mol
-
IUPAC Name: (R)-2-(1-hydroxyethyl)-6-fluoro-3-(3-fluorophenyl)-4H-chromen-4-one
-
Chemical Structure: Flavanone skeleton with fluorine substituents at the 6-position of the chromone ring and the meta-position of the phenyl ring; chiral center at the 2-carbon bearing the hydroxyethyl group.
This compound features an optically active center, and its nomenclature reflects the R-configuration as defined by Cahn-Ingold-Prelog priority rules. The fluorine atoms strategically alter electronic distribution and metabolic stability compared to non-fluorinated analogs, often enhancing target affinity or pharmacokinetic properties in research contexts.
PHYSICAL AND CHEMICAL PROPERTIES
| Property | Specification |
|---|---|
| Appearance | Off-white crystalline solid |
| Purity | Typically >98% (by HPLC) |
| Optical Purity | Confirmed enantiomeric excess (ee) > 99% |
| Solubility | Soluble in common organic solvents (e.g., DMSO, ethanol) |
| Stability | Stable under recommended storage conditions |
Fluorinated chromenones may exhibit enhanced thermal and chemical stability relative to unsubstituted counterparts. The presence of fluorine enhances lipophilicity, electronegativity effects, and metabolic resistance, which can be important parameters in medicinal chemistry exploration.
SYNTHETIC UTILITY
(R)-6-fluoro-3-(3-fluorophenyl)-2-(1-hydroxyethyl)-4H-chromen-4-one | CAS 1479107-10-2 serves as a valuable intermediate in synthetic chemistry workflows:
-
It can be incorporated into diversity-oriented synthesis (DOS) libraries to probe biological pathways.
-
The chiral center allows for enantiomer-specific studies, which are critical for pharmaceutical development and mechanistic enzymology.
-
Useful building block for structure-activity relationship (SAR) studies to explore how subtle changes influence potency, selectivity, and ADME (Absorption, Distribution, Metabolism, Excretion) profiles.
In medicinal chemistry programs, such fluorinated scaffolds are often exploited to improve target engagement, binding kinetics, and overall molecular performance.
APPLICATIONS IN RESEARCH & DEVELOPMENT
Medicinal Chemistry
Flavonoid-like structures, such as 4H-chromen-4-ones, are widely studied for their biological activity. The addition of fluorine atoms can improve:
-
Metabolic stability
-
Membrane permeability
-
Protein binding interactions
The R-enantiomer often displays distinct stereospecific properties critical to understanding chiral drug behavior.
Drug Discovery Screening
Used as a reference compound, synthetic intermediate, or scaffold in high-throughput screening (HTS) campaigns, this fluorinated chromenone can help identify novel hits or lead series for further development.
SAR Probing
The dual fluorine modification and chiral center make this compound a potent test case for SAR exploration, enabling chemists to assess how electronic and steric changes influence biological responses.
ANALYTICAL CHARACTERIZATION
At ResolveMass Laboratories, this compound is characterized using robust analytical techniques:
-
HPLC (High-Performance Liquid Chromatography) for purity analysis
-
Chiral HPLC to confirm enantiomeric excess
-
NMR (1H/13C/19F) to verify structural integrity
-
MS (Mass Spectrometry) for molecular weight confirmation
Comprehensive documentation, including Certificates of Analysis (CoA) and spectral data, is provided with every batch to support quality assurance and regulatory compliance in research settings.
SAFETY AND HANDLING
This compound is intended for research use only (RUO). It is not approved for human or veterinary use. Standard laboratory precautions should be followed:
-
Use appropriate PPE (gloves, goggles, lab coat)
-
Handle in a chemical fume hood
-
Avoid inhalation, ingestion, or skin contact
-
Dispose of waste according to institutional and regulatory guidelines
Detailed safety information is provided in the Safety Data Sheet (SDS).
WHY CHOOSE RESOLVEMASS LABORATORIES?
At ResolveMass Laboratories Inc., we specialize in high-purity research compounds trusted by chemists worldwide. Our commitment to:
-
Rigorous quality control
-
Reliable documentation
-
Chiral specialization
-
Fast turnaround and support
ensures you receive materials that meet your project’s exacting requirements.
Reviews
There are no reviews yet.