PRODUCT OVERVIEW
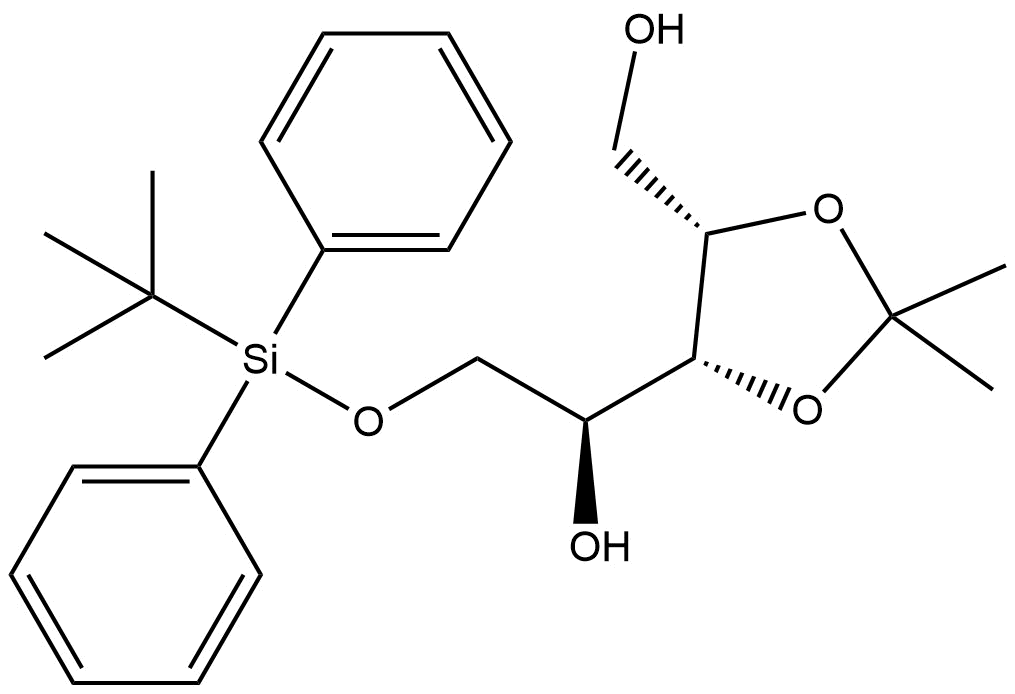
(S)-2-((tert-butyldiphenylsilyl)oxy)-1-((4R,5S)-5-(hydroxymethyl)-2,2-dimethyl-1,3-dioxolan-4-yl)ethan-1-ol | CAS 1006364-60-8 is a structurally complex, stereochemically defined chiral building block widely utilized in synthetic organic chemistry, particularly in medicinal chemistry, natural product synthesis, and complex molecule assembly. This compound features a protected diol moiety through tert-butyldiphenylsilyl (TBDPS) protection, combined with a 1,3-dioxolane ring system and a free hydroxymethyl group, making it highly adaptable for multi-step synthetic strategies.
With defined stereochemistry at both the 4R,5S positions within the dioxolane ring and the S-configuration at the ethan-1-ol side chain, this intermediate offers precise chirality for advanced synthesis applications. ResolveMass Laboratories Inc. offers this material with rigorous quality control, ensuring high purity and reliable performance for research and development usage.
CHEMICAL IDENTITY & STRUCTURE
-
Chemical Name: (S)-2-((tert-butyldiphenylsilyl)oxy)-1-((4R,5S)-5-(hydroxymethyl)-2,2-dimethyl-1,3-dioxolan-4-yl)ethan-1-ol
-
CAS Registry Number: 1006364-60-8
-
Molecular Formula: C₂₂H₃₀O₄Si
-
Molecular Weight: 430.62 g/mol (calculated)
-
Structural Features:
-
tert-butyldiphenylsilyl (TBDPS) protecting group: Enhances stability and orthogonality in protection strategies.
-
1,3-Dioxolane ring: A common protective motif for diols, contributing to stereochemical robustness.
-
Free hydroxymethyl group: Available for downstream functionalization.
-
Multiple stereocenters: (4R,5S) and S configurations facilitate enantioselective synthesis.
-
KEY FEATURES & BENEFITS
1. High Stereochemical Definition
This compound’s stereochemistry is precisely controlled, making it ideal for use in enantioselective syntheses and stereospecific transformations. The integrity of chirality is critical in fields such as drug discovery, where enantiomeric purity can influence biological activity and pharmacological profiles.
2. Orthogonal Protection Strategy
The TBDPS group offers robust protection of the secondary alcohol, tolerant to a broad range of reaction conditions (e.g., basic and mildly acidic environments) while remaining orthogonal to other common protecting groups. This allows synthetic chemists to selectively deprotect or modify functional groups at desired stages of a synthetic sequence.
3. Versatile Synthetic Intermediate
With a free primary hydroxymethyl group and a protected secondary alcohol, this compound serves as a versatile intermediate:
-
Nucleophilic substitution reactions
-
Oxidation to aldehydes or acids
-
Coupling reactions with electrophiles
-
Formation of ethers, esters, or carbamates
-
Elaboration to complex heterocycles
4. Compatibility with Modern Synthetic Techniques
The structural attributes of this compound make it compatible with:
-
Asymmetric catalysis
-
Organometallic additions
-
Cross-coupling reactions
-
Protecting group manipulations
Such versatility supports its use in the synthesis of complex natural products, medicinally relevant scaffolds, and advanced intermediates in total synthesis campaigns.
APPLICATIONS of (S)-2-((tert-butyldiphenylsilyl)oxy)-1-((4R,5S)-5-(hydroxymethyl)-2,2-dimethyl-1,3-dioxolan-4-yl)ethan-1-ol | CAS 1006364-60-8
Medicinal Chemistry & Drug Discovery
This chiral building block is frequently used in the preparation of bioactive molecules, including potential therapeutics where controlled stereochemistry and functional group placement are vital for binding specificity and activity.
Natural Product Synthesis
Due to its protected diol system and reactive hydroxymethyl functionality, it serves as a strategic intermediate in multi-step syntheses of natural products and analogues containing polyketide or carbohydrate elements.
Chemical Biology & Probe Development
Applicable in the construction of labeled or modified analogs used for mechanistic studies, biomarkers, and probe molecules in chemical biology research.
Advanced Materials
Chiral intermediates of this type may contribute to the production of optically active polymers or catalysts for enantioselective transformations.
PHYSICAL & CHEMICAL PROPERTIES
| Property | Value |
|---|---|
| Appearance | Off-white to pale yellow liquid/solid (dependent on batch and purity) |
| Molecular Formula | C₂₂H₃₀O₄Si |
| Molecular Weight | 430.62 g/mol |
| Stereochemistry | (S), (4R,5S) |
| Solubility | Soluble in common organic solvents (e.g., DCM, THF, toluene) |
| Stability | Stable under standard storage conditions, sensitive to strong acids and fluoride sources (due to TBDPS cleavage) |
QUALITY ASSURANCE & ANALYTICAL DATA
ResolveMass Laboratories Inc. guarantees comprehensive analytical characterization and quality control for this compound, including:
-
NMR Spectroscopy (¹H, ¹³C)
-
HPLC Purity Assessment
-
Mass Spectrometry
-
Chiral Purity Evaluation
Each batch is accompanied by a Certificate of Analysis (CoA) detailing spectral data, purity (%), and compliance with internal specifications. Custom analytical reports are available upon request for specialized requirements.
SAFETY & PRECAUTIONS
While specific safety data sheets (SDS) are provided with each shipment, standard laboratory safety protocols apply:
-
Avoid inhalation, ingestion, and contact with skin or eyes.
-
Use in well-ventilated areas.
-
Dispose of waste according to local regulations.
CONCLUSION
(S)-2-((tert-butyldiphenylsilyl)oxy)-1-((4R,5S)-5-(hydroxymethyl)-2,2-dimethyl-1,3-dioxolan-4-yl)ethan-1-ol (CAS 1006364-60-8) from ResolveMass Laboratories Inc. is a high-quality, chiral synthetic intermediate engineered for advanced organic synthesis applications. Its well-defined stereochemistry, versatile protecting group strategy, and compatibility with modern transformation techniques make it indispensable for medicinal chemistry, natural product synthesis, and chemical biology workflows.
![3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine | CAS 151266-23-8](https://resolvemass.ca/wp-content/uploads/2026/01/3-iodo-1H-pyrazolo34-dpyrimidin-4-amineCAS-151266-23-8-300x300.png)
Reviews
There are no reviews yet.