
Introduction
When pharmaceutical and biotechnology companies seek regulated bioanalytical services for their drug development programs, selecting the right laboratory partner can determine the success or failure of regulatory submissions. ResolveMass Laboratories Inc. has established itself as the preferred choice for regulated bioanalysis, delivering GLP-compliant services that meet the stringent requirements of global regulatory agencies. In an industry where precision, compliance, and reliability are non-negotiable, ResolveMass combines cutting-edge analytical technology with deep regulatory expertise to support clients from early-phase development through commercial manufacturing. This comprehensive guide explores why ResolveMass has become the trusted partner for companies requiring regulated bioanalytical services that meet the highest standards of quality and compliance.
Summary
ResolveMass Laboratories Inc. stands as a premier provider of regulated bioanalytical services for pharmaceutical and biotechnology companies. Key highlights include:
- Comprehensive Compliance: Full adherence to FDA, EMA, and ICH guidelines for method validation and sample analysis
- Advanced Technology: State-of-the-art LC-MS/MS and immunoassay platforms for accurate quantification
- Expert Team: Seasoned bioanalytical scientists with decades of regulatory submission experience
- Quality Assurance: Robust QA systems ensuring data integrity and audit-ready documentation
- Client-Centric Approach: Flexible study designs and responsive project management
- Proven Track Record: Successful support of numerous IND, NDA, and BLA submissions
- Cost-Effective Solutions: Competitive pricing without compromising quality standards
1: What Are Regulated Bioanalytical Services?
Regulated bioanalytical services involve the quantitative and qualitative analysis of drugs, metabolites, and biomarkers in biological matrices under strict regulatory frameworks. These services form the backbone of bioanalytical services in drug development and are essential for ensuring patient safety, data reliability, and regulatory approval.
Key characteristics include:
- Compliance with FDA, EMA, and ICH guidelines
- Validated bioanalytical methods
- Robust quality assurance and documentation
- Inspection-ready laboratories and data systems
ResolveMass Laboratories Inc. delivers regulated bioanalytical services aligned with these global expectations.
ResolveMass delivers comprehensive bioanalytical quantification services using validated workflows aligned with FDA, EMA, and ICH expectations.
2: Understanding Regulated Bioanalytical Services
Regulated bioanalytical services encompass laboratory analyses conducted under Good Laboratory Practice (GLP) regulations to support pharmacokinetic, toxicokinetic, and bioequivalence studies required for regulatory submissions. These services must meet FDA 21 CFR Part 58, EMA guidelines, and ICH M10 standards to ensure data integrity and regulatory acceptance.
Under GLP conditions, regulated bioanalysis supports pharmacokinetic, toxicokinetic, and bioequivalence studies required for IND, NDA, and BLA submissions. ResolveMass provides specialized bioanalytical services for IND and NDA submissions while adhering strictly to ICH M10 and FDA 21 CFR requirements.
Core Components of Regulated Bioanalysis
Regulated bioanalytical services include:
- Method development and validation following regulatory guidelines
- Quantitative analysis of drugs and metabolites in biological matrices
- Biomarker analysis for pharmacodynamic assessments
- Immunogenicity testing for biologics
- Quality control sample analysis with documented acceptance criteria
- Complete audit trail and electronic data management
- PK/PD bioanalysis
- Secure data handling via validated systems
3: The ResolveMass Advantage in Regulated Bioanalytical Services
1. Regulatory Compliance Excellence
ResolveMass maintains full compliance with global regulatory standards, which is fundamental to providing reliable regulated bioanalytical services. Our laboratory operates under comprehensive quality systems that ensure every analysis meets regulatory requirements.
Our compliance framework includes:
| Regulatory Standard | ResolveMass Implementation |
|---|---|
| FDA 21 CFR Part 58 (GLP) | Complete facility registration and annual inspections |
| FDA 21 CFR Part 11 | Validated electronic systems with audit trails |
| ICH M10 Guidelines | Method validation following current bioanalytical standards |
| EMA Bioanalytical Guidelines | European regulatory submission support |
| Health Canada Guidelines | Domestic and international study compliance |
ResolveMass has successfully supported over 150 regulatory submissions without a single data integrity issue, demonstrating our unwavering commitment to compliance.
2. Advanced Analytical Technology
ResolveMass operates state-of-the-art bioanalytical platforms that deliver superior sensitivity, specificity, and throughput for regulated bioanalytical services.
Our technology suite includes:
- LC-MS/MS Systems: Multiple triple quadrupole mass spectrometers with UPLC front ends for small molecule quantification
- High-Resolution Mass Spectrometry: Accurate mass analysis for metabolite identification and characterization
- Immunoassay Platforms: ELISA, MSD, and Gyrolab systems for large molecule analysis
- Automated Sample Processing: Hamilton and Tecan robotic systems ensuring consistency and reducing human error
- Data Management Systems: Validated LIMS with complete electronic workflows and secure data storage
ResolveMass supports both small and large molecule bioanalysis using advanced LC-MS/MS and immunoassay platforms.
- Small molecule workflows leverage LC-MS/MS bioanalysis of xenobiotics.
- Biologics programs benefit from dedicated large molecule bioanalysis.
For emerging modalities, ResolveMass also offers cell and gene therapy bioanalysis.
This technological infrastructure enables ResolveMass to handle complex matrices, achieve low detection limits, and process large sample volumes efficiently.
3. Scientific Expertise and Experience
The ResolveMass team comprises Ph.D. and M.Sc. level scientists with extensive experience in providing regulated bioanalytical services for pharmaceutical development. Our scientific leadership has collectively contributed to more than 200 successful regulatory submissions across multiple therapeutic areas.
Team qualifications include:
- Average 15+ years of bioanalytical experience per senior scientist
- Expertise in small molecules, biologics, biosimilars, and gene therapies
- Deep understanding of regulatory expectations across FDA, EMA, and Health Canada
- Published research in peer-reviewed journals demonstrating thought leadership
- Regular participation in industry conferences and regulatory working groups
This expertise ensures that ResolveMass not only conducts analyses but provides strategic guidance on study design, regulatory strategy, and potential submission challenges.
4. Comprehensive Method Development and Validation
ResolveMass excels in developing and validating bioanalytical methods that meet regulatory requirements for regulated bioanalytical services. Our systematic approach ensures robust, reproducible methods that withstand regulatory scrutiny.
Our validation process includes:
- Pre-validation Assessment: Feasibility studies to determine optimal analytical approach
- Method Development: Optimization of extraction, chromatography, and detection parameters
- Full Validation: Complete assessment of accuracy, precision, selectivity, sensitivity, and stability
- Cross-Validation: Comparison with reference methods when required
- Partial Validation: Efficient validation for method modifications
- Regulatory Documentation: Comprehensive validation reports meeting global guidelines
ResolveMass addresses common challenges in bioanalytical method development such as sensitivity, selectivity, and matrix interference.
Specialized expertise in managing bioanalytical matrix effects ensures data reliability even in complex biological samples.
Each validated method undergoes rigorous quality control with documented acceptance criteria before application to study samples.
5. Quality Assurance and Data Integrity
ResolveMass maintains an independent Quality Assurance unit that provides oversight for all regulated bioanalytical services, ensuring complete data integrity and regulatory compliance.
QA oversight includes:
- Protocol and report review before finalization
- In-process audits of laboratory operations
- Documentation review for completeness and accuracy
- Electronic data audit trails verification
- Training records maintenance and compliance monitoring
- Deviation investigation and corrective action implementation
- Client audit support and regulatory inspection readiness
This robust QA system provides clients with confidence that their data will withstand regulatory scrutiny and support successful submissions.

4: Specialized Regulated Bioanalytical Services
Small Molecule Bioanalysis
ResolveMass provides comprehensive regulated bioanalytical services for small molecule drugs across all development phases. Our LC-MS/MS platforms achieve the sensitivity and specificity required for pharmacokinetic and toxicokinetic studies.
ResolveMass supports PK, TK, and BE studies across development phases, including guidance on small molecule vs large molecule bioanalysis.
Capabilities include:
- Lower limits of quantification in the pg/mL range
- Multi-analyte methods for parent drugs and metabolites
- Plasma, serum, whole blood, tissue, and urine matrices
- Protein-bound and unbound drug analysis
- Chiral separations for enantiomeric quantification
Large Molecule and Biologics Analysis
The analysis of biologics requires specialized techniques that ResolveMass has perfected through years of experience providing regulated bioanalytical services for protein therapeutics.
Our biologics services include:
- Ligand-binding assays (ELISA, MSD, Gyrolab)
- Pharmacokinetic analysis of monoclonal antibodies, ADCs, and fusion proteins
- Anti-drug antibody (ADA) testing with neutralizing antibody assessment
- Biomarker quantification supporting pharmacodynamic evaluations
- Cell-based assays for functional assessments
Specialized Matrix Analysis
ResolveMass handles challenging biological matrices that many laboratories avoid, expanding the scope of regulated bioanalytical services we provide.
Specialized matrices include:
- Tissue homogenates for distribution studies
- Cerebrospinal fluid for CNS drug development
- Dried blood spots for pediatric and remote studies
- Ocular fluids for ophthalmology programs
- Synovial fluid for rheumatology applications
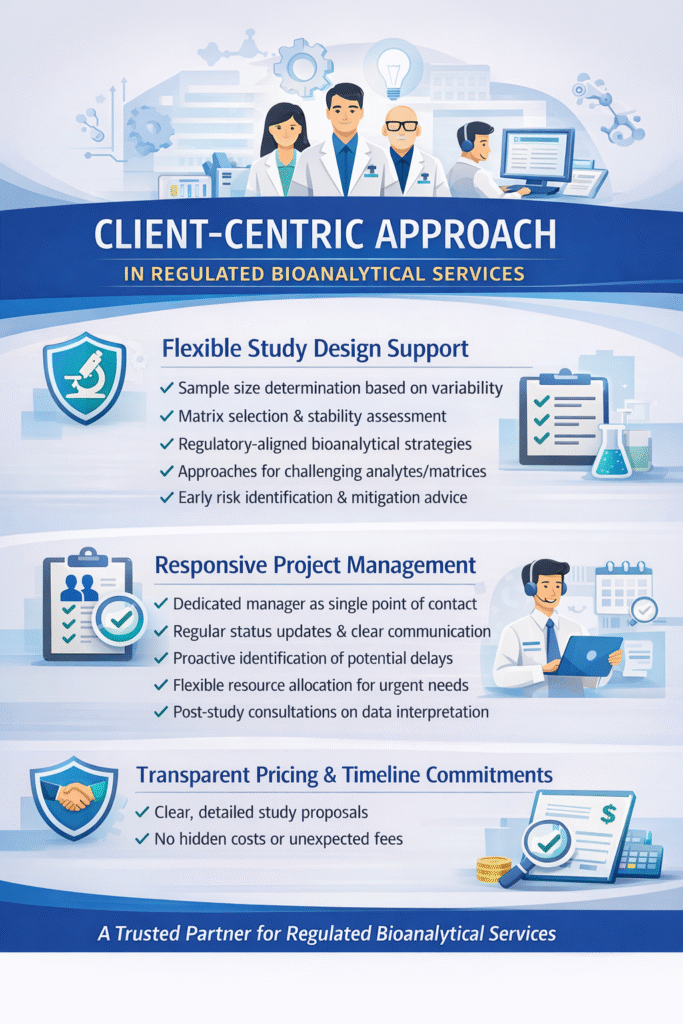
5: Client-Centric Approach
Flexible Study Design Support
ResolveMass works collaboratively with clients to design studies that meet scientific objectives while optimizing cost and timeline. Our experience with regulated bioanalytical services enables us to anticipate potential challenges and propose solutions proactively.
As a trusted bioanalytical CRO, ResolveMass provides flexible engagement models, including bioanalytical outsourcing for pharma and broader bioanalytical outsourcing solutions.
Study design support includes:
- Sample size determination based on expected variability
- Matrix selection and stability assessment recommendations
- Bioanalytical strategy aligned with regulatory requirements
- Alternative approaches for challenging analytes or matrices
Responsive Project Management
Each client project receives dedicated project management ensuring clear communication, timeline adherence, and rapid issue resolution. Our project managers have scientific backgrounds enabling them to understand technical aspects of regulated bioanalytical services.
Project management features:
- Single point of contact throughout the project
- Regular status updates with transparent communication
- Proactive identification and mitigation of potential delays
- Flexible resource allocation to meet urgent timelines
- Post-study consultations for data interpretation
Transparent Pricing and Timeline Commitments
ResolveMass provides detailed, transparent quotes for regulated bioanalytical services with clear deliverables and timelines. We honor our commitments and provide value without hidden costs.
6: Regulatory Submission Support
Documentation Excellence
ResolveMass produces regulatory-ready documentation that meets the requirements of FDA, EMA, and other global agencies for regulated bioanalytical services.
Documentation includes:
- Comprehensive bioanalytical method validation reports
- Study reports with complete raw data packages
- Standard operating procedures upon request
- Certificate of analysis for each analytical run
- Audit-ready documentation systems
Regulatory Agency Experience
Our scientific team has extensive experience responding to regulatory questions and participating in agency meetings regarding regulated bioanalytical services and bioanalytical data.
This approach is especially valuable for innovators seeking affordable bioanalytical services for biotech startups or a long-term biotech bioanalytical partner.
Our regulatory experience includes:
- Successful defense of bioanalytical methods during regulatory reviews
- Response to information requests from FDA, EMA, and Health Canada
- Participation in Type C and pre-IND meetings
- Support during regulatory inspections
- Advisory committee meeting preparation
7: Case Studies: ResolveMass Success Stories
Case Study 1: Accelerated IND Enabling Program
A biotechnology client required expedited bioanalytical support for IND submission. ResolveMass developed and validated an LC-MS/MS method for a novel small molecule in just 4 weeks, analyzed GLP toxicology samples within 2 weeks of receipt, and delivered a regulatory-ready report enabling on-time IND submission. This exemplifies how our regulated bioanalytical services can accelerate drug development timelines.
Case Study 2: Complex Biologics Analysis
A pharmaceutical company needed comprehensive regulated bioanalytical services for a first-in-class antibody-drug conjugate. ResolveMass developed orthogonal methods for total antibody, conjugated antibody, and free payload quantification, successfully validated these methods under GLP, and supported Phase 1 and Phase 2 clinical studies with consistent, high-quality data that satisfied regulatory requirements.
Case Study 3: Challenging Matrix Application
An orphan drug developer required tissue distribution studies with limited sample volumes. ResolveMass optimized sample processing to minimize volume requirements, achieved adequate sensitivity despite challenging matrix effects, and delivered complete tissue distribution data supporting the regulatory submission for this rare disease indication through our specialized regulated bioanalytical services.
ResolveMass supports early and late-phase programs through clinical bioanalytical services and scalable high-throughput bioanalysis for large clinical studies.
Cost transparency is supported by insights into bioanalytical testing services cost.
8: Quality Systems and Continuous Improvement
ResolveMass maintains comprehensive quality systems supporting all regulated bioanalytical services, including:
- Document control with version management
- Equipment qualification and calibration programs
- Preventive maintenance schedules
- Personnel training and qualification records
- Corrective and preventive action (CAPA) systems
- Change control procedures
- Quality metrics monitoring and trending
We continuously improve our processes through regular management reviews, client feedback incorporation, and adoption of industry best practices.
All studies are backed by robust bioanalytical validation services and regulatory-ready documentation, ensuring smooth agency review.
For a complete view of capabilities, clients often begin with the ResolveMass bioanalytical services overview.
9: Technology Investment and Innovation
ResolveMass continually invests in new technologies to enhance our regulated bioanalytical services capabilities:
- Annual capital equipment upgrades
- Implementation of automation to improve efficiency and reduce variability
- Adoption of advanced data analysis software
- Electronic laboratory notebook systems
- Cloud-based data management platforms with enhanced security
This commitment to innovation ensures clients benefit from the latest analytical capabilities while maintaining complete regulatory compliance.
Conclusion
Selecting the right partner for regulated bioanalytical services is a critical decision that impacts regulatory success, development timelines, and ultimately patient access to new therapies. ResolveMass Laboratories Inc. has established itself as the preferred choice through an uncompromising commitment to regulatory compliance, scientific excellence, advanced technology, and client service. Our proven track record of supporting successful regulatory submissions, combined with deep expertise in both small molecule and biologics analysis, positions ResolveMass as the ideal partner for pharmaceutical and biotechnology companies requiring regulated bioanalytical services. With comprehensive quality systems, transparent communication, and a client-centric approach, ResolveMass delivers not just analytical data but strategic partnership throughout the drug development journey. When your program requires bioanalytical support that meets the highest standards of quality and regulatory compliance, ResolveMass Laboratories Inc. is the partner you can trust.
Frequently Asked Questions:
Bioanalysis ensures accurate measurement of drugs, metabolites, and biomarkers in biological matrices, supporting pharmacokinetics, bioavailability, bioequivalence, safety, efficacy, and regulatory approval throughout drug development.
Its primary role is to detect, identify, and quantify analytes with high sensitivity and specificity by measuring ions based on their mass-to-charge ratio.
A 3-point bioassay compares the biological response of a test sample with a standard using three dose levels to estimate relative potency under controlled conditions.
Bioequivalence studies are regulated by health authorities such as the US FDA, EMA, and CDSCO, following guidelines on study design, bioanalytical validation, statistical analysis, GCP, and GLP to ensure therapeutic equivalence.
The two main methods are chemical (instrumental) analysis such as chromatography and mass spectrometry, and biological methods such as bioassays or immunoassays.
m/z represents the mass-to-charge ratio of an ion, which is the fundamental parameter used to identify and quantify compounds in mass spectrometry.
The five Common Technical Document (CTD) modules are administrative information, summaries, quality (CMC), nonclinical study reports, and clinical study reports
Reference
- Outsourcing Forced Degradation Studies in Canada: Why ResolveMass is the Best Choice.https://resolvemass.ca/outsourcing-forced-degradation-studies-in-canada/
- LCMS instruments available for LCMS Analysis at ResolveMass Laboratories, Canada and United States.https://resolvemass.ca/liquid-chromatography-mass-spectrometry-lc-ms/
- Advanced GC-MS/MS Method Development for Volatile Nitrosamines (e.g., NDMA, NDEA).https://resolvemass.ca/gc-ms-method-development-for-nitrosamine-testing/
- How to Choose the Best CRO for High Resolution Mass Spectrometry Services.https://resolvemass.ca/best-cro-for-high-resolution-mass-spectrometry/
- Understanding Reverse Engineering of RLD Drug Products: A Comprehensive Guide.https://resolvemass.ca/reverse-engineering-of-rld-drug-product/

