Introduction:
Reverse engineering of PLGA polymer in Lupron Depot represents a scientifically rigorous and commercially critical pathway for pharmaceutical companies developing generic versions of this benchmark long-acting injectable formulation. Since its FDA approval in 1989, Lupron Depot has become the gold standard against which modern long-acting release (LAR) products are compared, with annual U.S. market sales reaching $580 million in 2014. Despite patent expiration, no generic version has been approved in the United States, primarily due to the formulation’s complexity and the stringent analytical requirements for demonstrating bioequivalence.
The reverse engineering of PLGA polymer in Lupron Depot is essential because the 1-month formulation represents a sophisticated microsphere system where leuprolide acetate—a water-soluble gonadotropin-releasing hormone (GnRH) agonist peptide—is encapsulated in poly(lactic-co-glycolic acid) microspheres along with gelatin. The formulation achieves sustained drug release over 30 days through a carefully engineered combination of PLGA properties, manufacturing processes, and excipient interactions. Understanding these intricate relationships requires specialized analytical capabilities that can accurately characterize each component and critical quality attribute.
For a deeper understanding of PLGA-based microsphere systems, see our detailed PLGA microsphere case study:
🔗 https://resolvemass.ca/plga-microsphere-case-study/
For generic drug developers pursuing Abbreviated New Drug Application (ANDA) approval under the 505(j) pathway, achieving qualitative (Q1) and quantitative (Q2) sameness to the reference listed drug (RLD) is mandatory. This necessitates comprehensive reverse engineering of PLGA polymer in Lupron Depot to establish identical formulation composition, matching polymer characteristics, and equivalent performance attributes—expertise that ResolveMass Laboratories Inc. has developed through years of specialized work in complex generic development.
Summary
Reverse engineering of PLGA polymer in Lupron Depot involves detailed analytical characterization of the biodegradable poly(lactic-co-glycolic acid) copolymer to determine composition (75:25 lactic acid to glycolic acid ratio), molecular weight (Mw ~13.0 kDa, Mn ~8.7 kDa), polydispersity index (~1.5), end-group chemistry (carboxylic acid terminated), and degradation behavior for developing equivalent generic formulations.
Key Takeaways:
- Lupron Depot uses PLGA microspheres (75:25 LA/GA ratio, Mw 12-14 kDa) as the delivery system for leuprolide acetate
- Reverse engineering of PLGA polymer in Lupron Depot requires extraction, purification, and comprehensive characterization using GPC, NMR, DSC, and titration methods
- PLGA characterization reveals critical parameters: 88.3% PLGA content, acid number ~12.9 mg KOH/g, and glass transition temperature ~48.6°C
- Multiple analytical methods must be employed to achieve accurate results, including solvent extraction, molecular weight determination, and compositional analysis
- Product attributes include 11.4 μm median particle size, <0.5% residual moisture, <1 ppm residual methylene chloride, and zero-order release kinetics after initial burst
- Regulatory compliance demands comprehensive documentation matching Q1 (qualitative) and Q2 (quantitative) sameness to the reference listed drug
- ResolveMass Laboratories Inc. provides specialized expertise in PLGA polymer analysis and reverse engineering for pharmaceutical development
1: What is PLGA and Why is it Used in Lupron Depot?
PLGA (poly(lactic-co-glycolic acid)) is an FDA-approved biodegradable copolymer that degrades hydrolytically into lactic acid and glycolic acid—both naturally occurring metabolites—making it ideal for sustained drug release in Lupron Depot, where it controls peptide release over one to six months depending on formulation.
PLGA Polymer Fundamentals
Poly(lactic-co-glycolic acid) serves as the cornerstone technology enabling Lupron Depot’s extended-release performance. The polymer offers several critical advantages:
- Biocompatibility: PLGA degrades into non-toxic, naturally occurring metabolites
- Tunable degradation rates: Controlled by lactide:glycolide ratio and molecular weight
- FDA approval history: Decades of documented safety in clinical applications
- Versatile manufacturing compatibility: Suitable for various microsphere preparation methods including solvent evaporation and spray drying
- Predictable release kinetics: Enables precise dosing schedules from 1 to 6 months
Practical guidance on solvent selection and handling is discussed in dissolving PLGA in solvents for analytical and formulation studies:
🔗 https://resolvemass.ca/dissolving-plga-in-solvents/
Lupron Depot’s PLGA Composition
The 7.5 mg 1-month Lupron Depot formulation utilizes specific PLGA characteristics optimized for monthly dosing:
Chamber 1 (Microsphere Powder) Contains:
- 7.5 mg leuprolide acetate (8.5 wt%)
- 66.2 mg PLGA (75:25 LA/GA ratio, 88.3 wt%)
- 1.3 mg gelatin (Type B, ~300 bloom, 1.5 wt%)
- 13.2 mg D-mannitol (15 wt%)
Chamber 2 (Diluent) Contains:
- 5 mg carboxymethylcellulose sodium (0.5%)
- 50 mg mannitol (5%)
- 1 mg polysorbate 80 (0.1%)
- Water for injection (94.4%)
- Glacial acetic acid (pH control)
The PLGA matrix provides protection from enzymatic degradation while enabling sustained release through a combination of diffusion and polymer erosion mechanisms, reducing injection frequency from daily to monthly administration.
2: The Science Behind Reverse Engineering of PLGA Polymer in Lupron Depot
Reverse engineering of PLGA polymer in Lupron Depot involves systematic polymer extraction from microspheres, followed by comprehensive characterization using GPC (molecular weight: Mw ~13.0 kDa, Mn ~8.7 kDa, PDI ~1.5), ¹H NMR (LA/GA ratio: 74.3/25.7), titration (acid number: ~12.9 mg KOH/g), and DSC (Tg: ~48.6°C) to establish complete polymer specifications.
Critical PLGA Parameters Characterized in Lupron Depot
| Parameter | Analytical Technique | Lupron Depot Values | Impact on Performance |
|---|---|---|---|
| Weight-Average Molecular Weight (Mw) | Gel Permeation Chromatography (GPC) | ~13.0 kDa | Controls degradation duration and mechanical strength |
| Number-Average Molecular Weight (Mn) | GPC | ~8.7 kDa | Influences initial polymer properties |
| Polydispersity Index (PDI) | GPC | ~1.5 | Indicates molecular weight distribution uniformity |
| Lactide:Glycolide Ratio | ¹H NMR Spectroscopy | 74.3:25.7 (75:25) | Determines hydrophobicity and degradation rate |
| PLGA Content | Quantitative NMR | 87.0 ± 0.3% | Confirms formulation composition |
| Acid Number | Organic Phase Titration | 12.9 mg KOH/g | Indicates carboxylic acid end-group presence |
| Glass Transition Temperature (Tg) | Modulated DSC | 48.6 ± 0.1°C | Affects storage stability and processing |
| Residual Moisture | Karl Fischer Titration | 0.44 ± 0.10% | Critical for long-term stability |
| Residual Solvent (DCM) | Gas Chromatography | <1 ppm | Safety and quality indicator |
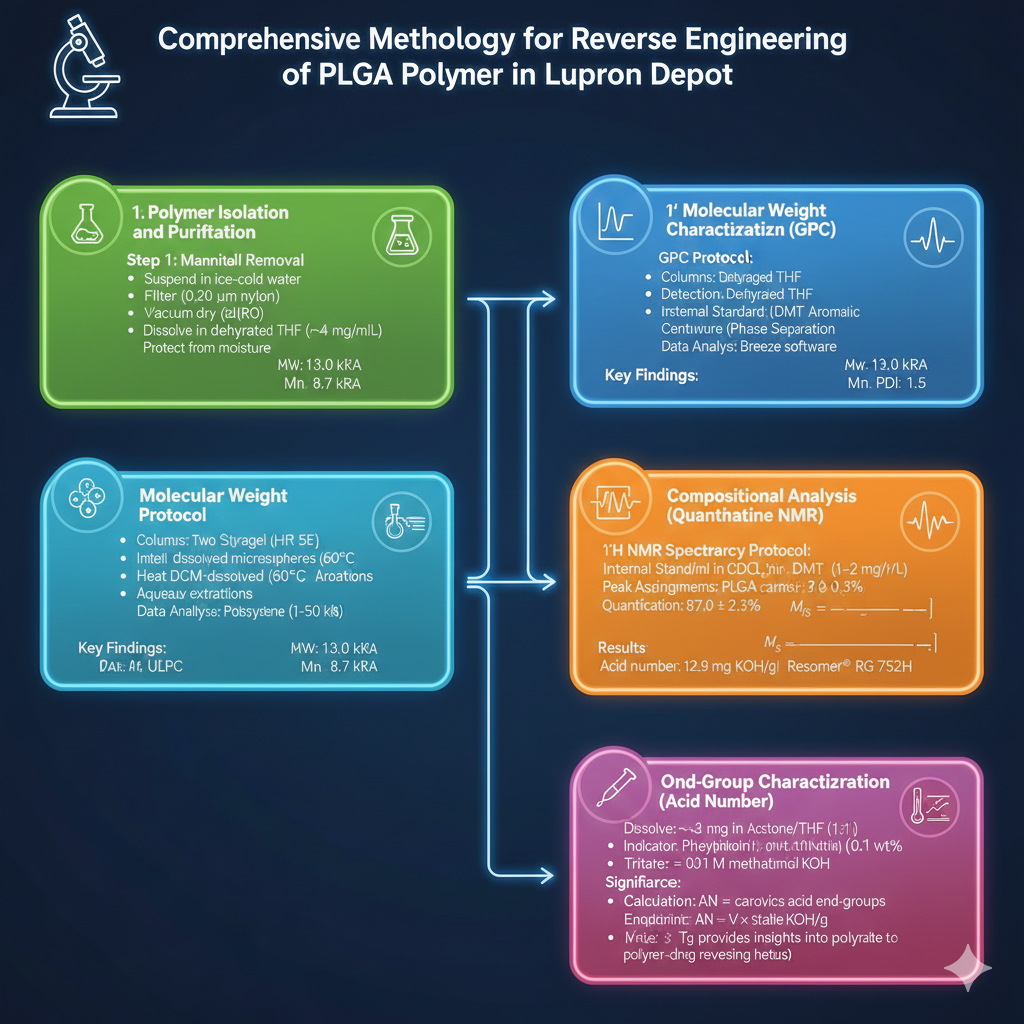
Comprehensive Methodology for Reverse Engineering of PLGA Polymer in Lupron Depot
Phase 1: Polymer Isolation and Purification
The reverse engineering of PLGA polymer in Lupron Depot begins with careful polymer extraction to avoid degradation:
Step 1: Mannitol Removal
- Suspend formulation powder in ice-cold water
- Ice temperature prevents PLGA hydrolytic degradation
- Filter microspheres using 0.20 μm nylon membrane under vacuum
- Wash with additional 5 mL ddH₂O to remove surface-bound mannitol
- Vacuum dry at room temperature until constant weight achieved
Step 2: PLGA Dissolution
- Dissolve mannitol-free microspheres in dehydrated tetrahydrofuran (THF)
- THF dehydration using 3-Å molecular sieves prevents polymer degradation
- Maintain concentration at approximately 4 mg/mL for GPC analysis
- Protect samples from moisture throughout handling
Step 3: Drug and Gelatin Removal
- For gelatin extraction: heat DCM-dissolved microspheres with water to 60°C
- Perform multiple aqueous extractions to ensure complete removal
- Centrifuge at appropriate speeds to achieve phase separation
- Verify complete drug removal through UPLC analysis
Moisture sensitivity during these steps is a major risk factor for PLGA degradation, highlighting the importance of stability control discussed in PLGA formulation stability: https://resolvemass.ca/plga-formulation-stability/
Phase 2: Molecular Weight Characterization
Gel Permeation Chromatography (GPC) Protocol:
The reverse engineering of PLGA polymer in Lupron Depot revealed specific molecular weight characteristics through optimized GPC methods:
- Column Setup: Two styragel columns (HR 1 and HR 5E) for optimal separation
- Mobile Phase: Dehydrated THF at controlled flow rate
- Detection: Refractive index detector (2414 RI detector)
- Calibration: Polystyrene standards ranging 1,000-50,000 Da
- Data Analysis: Breeze software for Mw, Mn, and PDI calculations
Key Findings:
- Mw: 13.0 kDa (matches published raw polymer specifications of 12.1-14 kDa)
- Mn: 8.7 kDa (indicates narrow molecular weight distribution)
- PDI: 1.5 (slightly lower than published 1.81, suggesting careful manufacturing)
These molecular weight values represent the finished product and confirm minimal degradation occurred during the double emulsion solvent evaporation manufacturing process.
Phase 3: Compositional Analysis by Quantitative NMR
¹H NMR Spectroscopy Protocol:
Quantitative NMR provides definitive identification of PLGA composition in Lupron Depot:
Sample Preparation:
- Dissolve mannitol-free microspheres in CDCl₃ at 15-20 mg/mL
- Add dimethyl terephthalate (DMT) as internal standard at 1.0-2.0 mg/mL
- Ensure complete dissolution before analysis
Peak Assignments:
- Methyl (-CH₃) protons from lactic acid units
- Methylene (-CH₂) protons from glycolic acid units
- DMT aromatic protons for quantification
Quantification Equation:
M_s = M_IS × (Mw_s/Mw_IS) × (nH_IS/nH_s) × (P_IS/P_s) × (A_s/A_IS)Where:
- M = mass
- Mw = molecular weight
- nH = number of contributing protons
- P = purity
- A = peak area
- Subscripts: s = sample (LA or GA), IS = internal standard
Results from Reverse Engineering:
- LA/GA ratio: 74.3/25.7 (closely matches expected 75/25)
- PLGA content: 87.0 ± 0.3% (consistent with labeled 88.3%)
- Method provides simultaneous composition and content determination
Phase 4: End-Group Characterization
Acid Number Determination by Organic Phase Titration:
The reverse engineering of PLGA polymer in Lupron Depot includes critical end-group analysis:
Titration Protocol:
- Dissolve ~10 mg LD in 5 mL dehydrated acetone/THF (1:1, v/v)
- Add 0.1 wt% phenolphthalein methanol solution as indicator
- Titrate immediately with 0.01 M methanolic KOH to stable pink endpoint
- Calculate acid number: AN = (V_sample × N_KOH × MW_KOH) / Weight_PLGA
Significance of Results:
- Acid number: 12.9 mg KOH/g PLGA
- High value indicates carboxylic acid end-groups (not ester-capped)
- Comparable to Resomer® RG 752H (14.3 mg KOH/g, Mw 13 kDa)
- Acid end-groups enhance drug-polymer interactions
- Confirms PLGA is synthesized without ester end-capping
This finding is critical because end-group chemistry significantly affects:
- Drug-polymer electrostatic interactions
- Microclimate pH during degradation
- Release kinetics and mechanism
- Product stability
Phase 5: Thermal Characterization
Modulated Differential Scanning Calorimetry (mDSC):
Glass transition temperature (Tg) provides insights into polymer-drug interactions:
Experimental Conditions:
- Sample size: 3-5 mg LD microspheres
- Temperature range: -20°C to 90°C
- Heating rate: 3°C/min
- Cycle: Heat/cool/heat protocol
- Analysis: Tg taken as midpoint of reversing heat event
Key Finding:
- Tg: 48.6 ± 0.1°C (significantly higher than pure PLGA)
- Elevated Tg indicates strong peptide-polymer interactions
- High drug loading (10%) contributes to Tg increase
- Leuprolide acts as anti-plasticizer, increasing chain stiffness
Learn more about degradation pathways and shelf-life considerations in PLGA formulation stability studies:
🔗 https://resolvemass.ca/plga-formulation-stability/Reverse engineering strategies for PLGA-based microspheres are closely linked to formulation design principles outlined in PLGA microsphere formulation development: https://resolvemass.ca/plga-microsphere-formulation/
3: Analytical Challenges in Reverse Engineering of PLGA Polymer in Lupron Depot
Quick Answer: Major challenges include achieving complete drug extraction without polymer degradation, preventing moisture-induced hydrolysis during analysis, accurately separating gelatin from PLGA without interference, selecting appropriate GPC standards for molecular weight calibration, and avoiding artifactual degradation during multi-step extraction procedures.
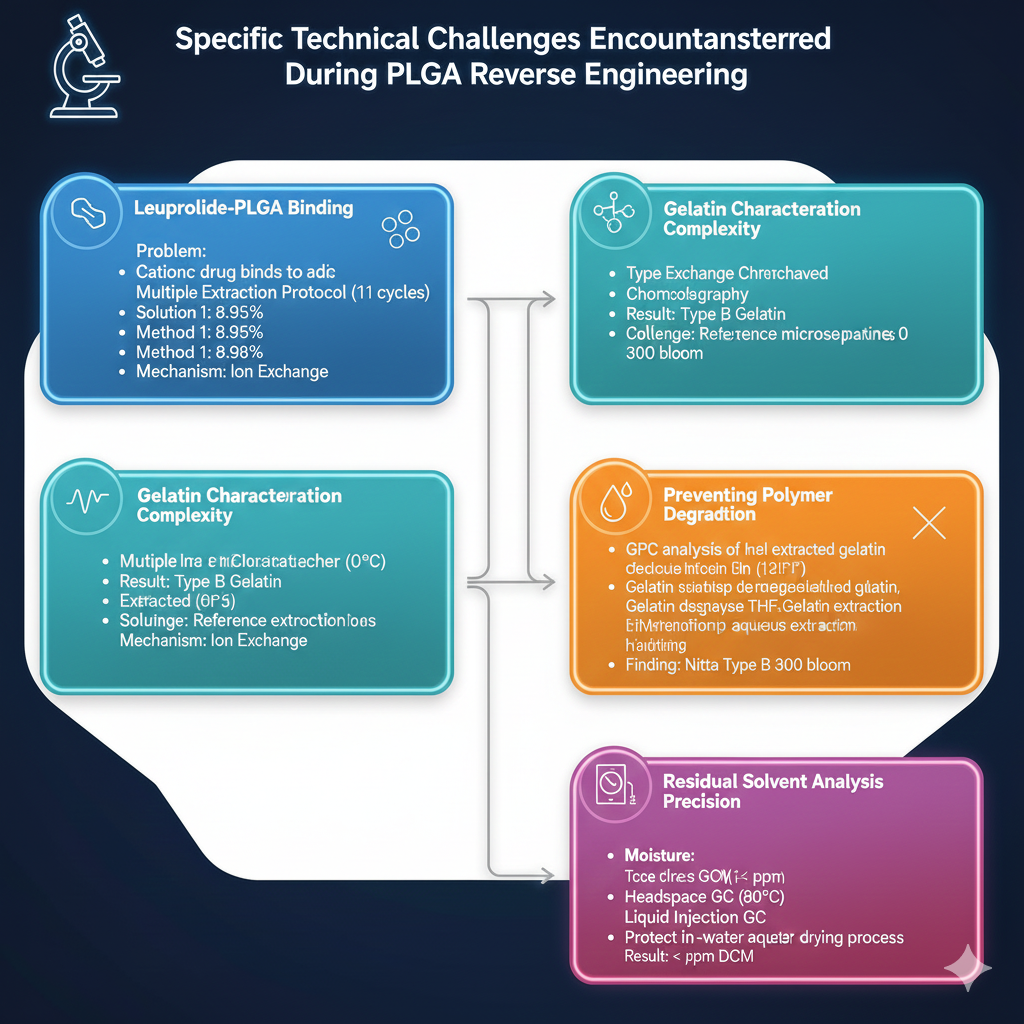
Specific Technical Challenges Encountered
Challenge 1: Leuprolide-PLGA Binding
Cationic leuprolide binds strongly to negatively charged PLGA carboxylic acid end-groups:
- Problem: Single extraction insufficient for complete drug recovery
- Solution: Multiple extraction protocol required
- Method 1 (single extraction): 8.31 ± 0.05 wt% recovery
- Method 2 (11 extractions): 8.95 ± 0.31 wt% recovery
- Method 3 (amino acid analysis): 8.89 ± 0.13 wt% recovery
- Mechanism: Acetate counterion less acidic than PLGA end-group, leading to ion exchange
- Implication: Standard extraction methods may underestimate drug loading
Challenge 2: Gelatin Characterization Complexity
Gelatin poses unique analytical challenges in reverse engineering of PLGA polymer in Lupron Depot:
Type Identification:
- Ion exchange chromatography differentiates Type A (pI ~9) from Type B (pI ~5)
- Required purification: Molecular cut-off filtration (10 kDa) to remove leuprolide
- Multiple extraction/concentration cycles needed
- Result: Type B gelatin confirmed (retention time ~4.2 min)
Molecular Weight Distribution:
- Bloom number relates to molecular weight but requires extensive sample
- Alternative approach: GPC analysis of extracted gelatin
- Challenge: Gelatin degradation during microsphere manufacturing
- Solution: Prepare reference microspheres with various gelatin grades
- Finding: Nitta Type B 300 bloom gelatin matched LD profile
Challenge 3: Preventing Polymer Degradation
PLGA is highly susceptible to degradation during analysis:
- Moisture sensitivity: Use ice-cold water for initial processing
- Solvent selection: Dehydrate THF with molecular sieves before GPC
- Temperature control: Maintain low temperatures during extraction
- Handling time: Minimize exposure to aqueous conditions
- Storage: Protect dried samples from humidity
Challenge 4: Residual Solvent Analysis Precision
Detecting trace levels of methylene chloride requires optimized methods:
- Matrix complexity: PLGA microspheres in DMSO
- Sensitivity requirements: Detection below 1 ppm necessary
- Dual method approach:
- Headspace GC: 80°C, 20 min equilibration
- Liquid injection GC: Direct injection method
- Result: <1 ppm residual DCM (far below 100 ppm literature value)
- Significance: Demonstrates excellence of in-water drying process
Leuprolide exhibits strong electrostatic binding to acid-terminated PLGA, complicating extraction and quantification. Drug loading strategies and mitigation approaches are discussed in PLGA drug loading: https://resolvemass.ca/plga-drug-loading/
Additional approaches to improve API dispersion and performance are covered in PLGA solubility enhancement: https://resolvemass.ca/plga-solubility-enhancement/
4: Regulatory Considerations for Reverse Engineering of PLGA Polymer in Lupron Depot
FDA requires Q1 (qualitative) and Q2 (quantitative) sameness for ANDA approval under 505(j) pathway, demanding comprehensive documentation of PLGA molecular weight, LA/GA ratio, end-group identity, impurity profile, and manufacturing consistency, with all analytical methods validated per ICH guidelines.
FDA Expectations for Generic PLGA Depot Products
The regulatory pathway for reverse engineering of PLGA polymer in Lupron Depot follows specific FDA guidance:
Q1 (Qualitative) Sameness Requirements:
- Same polymer type (PLGA, not PLA or PGA alone)
- Same copolymer ratio (75:25 LA/GA)
- Same end-group chemistry (carboxylic acid terminated)
- Same excipients (gelatin type B, mannitol, diluent components)
- Same dosage form (dual-chamber pre-filled syringe)
Q2 (Quantitative) Sameness Requirements:
- PLGA molecular weight within acceptable ranges
- Drug loading matching RLD (8.5 wt%)
- Excipient levels matching labeled amounts
- Particle size distribution comparable to RLD
- Similar impurity profiles and residual solvents
For ANDA approval, FDA expects demonstration of Q1 (qualitative) and Q2 (quantitative) sameness for complex injectables. Regulatory strategy and analytical expectations are detailed in Q1/Q2 polymer equivalence assessment: https://resolvemass.ca/q1-q2-polymer-equivalence-assessment/
Critical Documentation for ANDA Submission
Polymer Specifications Must Include:
| Specification | Test Method | Acceptance Criteria (Example) |
|---|---|---|
| Molecular Weight (Mw) | GPC vs. polystyrene standards | 12.0-14.0 kDa |
| Molecular Weight (Mn) | GPC vs. polystyrene standards | 8.0-9.5 kDa |
| Polydispersity Index | GPC calculation | 1.3-1.7 |
| LA/GA Ratio | ¹H NMR | 73-77:27-23 |
| Acid Number | Titration | 11-15 mg KOH/g |
| Residual Lactide | GC or HPLC | NMT 2.0% |
| Residual Glycolide | GC or HPLC | NMT 2.0% |
| Heavy Metals | ICP-MS | NMT 20 ppm |
| Residual Solvents | GC headspace | Meets ICH Q3C limits |
Analytical Method Validation Requirements:
- Specificity/selectivity demonstration
- Linearity across working range
- Accuracy/recovery studies
- Precision (repeatability and intermediate precision)
- Range establishment
- Detection and quantitation limits
- Robustness/ruggedness evaluation
Comparative Analysis Strategy
Successful reverse engineering of PLGA polymer in Lupron Depot requires head-to-head comparison:
Multi-Lot RLD Testing:
- Analyze minimum 3-5 lots from different manufacturing dates
- Establish ranges for all critical parameters
- Document batch-to-batch variability
- Justify generic product specifications based on RLD data
In Vitro Release Testing:
- USP apparatus appropriate for microspheres
- Sample-and-separate method in PBST (pH 7.4, 0.02% Tween 80)
- Temperature: 37°C with gentle agitation
- Sampling timepoints: Days 1, 3, 7, then weekly
- Acceptance criteria based on RLD release profile
Statistical Comparison:
- One-sample t-tests comparing generic to RLD labeled values
- Two-sample t-tests for direct comparisons
- Significance level: α = 0.05 (95% confidence interval)
- Equivalence testing for release profiles (f₂ similarity factor)
5: Case Study: Complete Reverse Engineering of PLGA Polymer in Lupron Depot 1-Month Formulation
Comprehensive reverse engineering of the 1-month Lupron Depot formulation confirmed 11.4 μm median particle size, zero-order release after 23% initial burst, Type B gelatin (~300 bloom), PLGA 75:25 LA/GA ratio with Mw 13.0 kDa, and exceptional purity with <1 ppm residual solvent—establishing complete product specifications for generic development.
Complete Formulation Characterization Results
Microsphere Composition (Chamber 1):
| Component | Labeled Value | Measured Value | Method | Statistical Significance |
|---|---|---|---|---|
| Leuprolide Acetate | 8.5 wt% | 8.89 ± 0.13 wt% | Amino acid analysis | p > 0.05 (NS) |
| PLGA | 88.3 wt% | 87.0 ± 0.3 wt% | Quantitative NMR | Close match |
| Gelatin | 1.5 wt% | 1.55 ± 0.08 wt% | Amino acid analysis | p > 0.05 (NS) |
| D-Mannitol | 15.0 wt% | 15.63 ± 0.43 wt% | Colorimetric assay | p > 0.05 (NS) |
Diluent Composition (Chamber 2):
| Component | Labeled Value | Measured Value | Method |
|---|---|---|---|
| Water | 94.4 wt% | 94.55 ± 0.01 wt% | Gravimetric |
| D-Mannitol | 5.0 wt% | 4.42 ± 0.07 wt% | Colorimetric assay |
| Na-CMC | 0.5 wt% | By difference | Viscometry correlation |
| Polysorbate 80 | 0.1 wt% | 0.116 ± 0.003 wt% | Fluorescence (bis-ANS) |
| Glacial Acetic Acid | pH control | pH 6.0-7.0 measured | pH meter |
Product Performance Attributes
Particle Size Distribution:
- Volume-median diameter (d₀.₅): 11.4 ± 0.5 μm
- d₀.₁ (10% volume below): 3.8 ± 0.2 μm
- d₀.₉ (90% volume below): 30.0 ± 0.6 μm
- Distribution: Narrow, unimodal
- Morphology: Spherical microspheres (confirmed by SEM)
- Majority: <20 μm diameter
Quality and Purity:
- Residual moisture: 0.44 ± 0.10% (Karl Fischer)
- Residual methylene chloride: <1 ppm (dual GC methods)
- Glass transition temperature: 48.6 ± 0.1°C (mDSC)
Release Kinetics Profile:
- Initial burst release (Day 1): 22.8 ± 0.4%
- Release pattern: Zero-order after Day 3
- Time to 50% release (t₅₀): 12.3 ± 0.2 days
- 80% release: Day 35
- Complete release: Day 49 (7 weeks)
- Release medium: PBST (pH 7.4, 0.02% Tween 80, 0.02% NaN₃)
Gelatin Characterization Breakthrough
The reverse engineering of PLGA polymer in Lupron Depot achieved definitive gelatin identification:
Type Identification via Ion Exchange:
- Separation: TSKgel SP-NPR cation exchange column
- Detection: UV 220 nm at 50°C column temperature
- Gradient elution: pH 3 citric acid buffer to pH 11.5 phosphate buffer
- Result: Major peak at 4.2 min retention time
- Conclusion: Type B gelatin (Type A peak appears at 13.5 min)
Molecular Weight Distribution Analysis:
- Method: GPC with TSKgel UP-SW3000 column
- Mobile phase: Phosphate buffer (pH ~7)
- Standards: Gel filtration markers (29-700 kDa)
- Sample preparation: Extraction, purification, molecular cut-off filtration
- Finding: MW distribution matched Nitta Type B 300 bloom gelatin
- Supporting evidence: Nitta B 300 is low endotoxin, injection-grade
- Literature confirmation: Used in inventor’s publications
Functional Role:
- Original purpose: Increase encapsulation efficiency (viscosity enhancement)
- Key discovery: Cooling primary emulsion (increased viscosity) is actual mechanism
- Gelatin remains in formulation for historical/consistency reasons
- May influence microsphere properties and stability
The formulation of potent peptide APIs using PLGA systems is further explored in formulating highly potent APIs using PLGA microspheres: https://resolvemass.ca/formulating-highly-potent-apis-using-plga-polylactic-co-glycolic-acid-microspheres/
Critical Manufacturing Insights
The reverse engineering of PLGA polymer in Lupron Depot revealed key process characteristics:
Solvent Removal Excellence:
- Expected residual DCM: <100 ppm (per literature)
- Measured residual DCM: <1 ppm
- Method: In-water drying during manufacturing
- Significance: Demonstrates sophisticated process control
- Implication: Generic developers must achieve similar solvent removal efficiency
Moisture Control:
- Residual moisture: <0.5%
- Critical for PLGA stability (prevents hydrolytic degradation)
- Achieved through careful lyophilization
- Required for acceptable shelf life
Microsphere Characteristics:
- PVA removal: Extensive washing (>1 L water) required
- Manufacturing method: W₁/O/W₂ double emulsion solvent evaporation
- Size control: <90 μm sieve removes large particles
- Surface: Smooth, non-porous (early stage microspheres)
- Core-shell potential: Investigated for zero-order release
6: How ResolveMass Laboratories Inc. Supports Reverse Engineering of PLGA Polymer in Lupron Depot Projects
ResolveMass Laboratories Inc. provides comprehensive analytical services for reverse engineering of PLGA polymer in Lupron Depot including polymer extraction, multi-technique characterization (GPC, NMR, DSC, titration), gelatin typing, particle analysis, release testing, method validation, and regulatory documentation support using state-of-the-art instrumentation and PhD-level scientific expertise.
Specialized Analytical Capabilities
ResolveMass Laboratories Inc. offers the complete suite of techniques required for successful reverse engineering of PLGA polymer in Lupron Depot:
Polymer Characterization Services
Molecular Weight Analysis:
- Gel Permeation Chromatography (GPC/SEC)
- Multi-detector systems (RI, UV, light scattering)
- Multiple column sets for different MW ranges
- Polystyrene and PLGA standard calibrations
- Dehydrated solvent systems for moisture-sensitive polymers
- Comprehensive MW distribution analysis (Mw, Mn, Mz, PDI)
Compositional Analysis:
- Quantitative ¹H NMR and ¹³C NMR spectroscopy
- High-field instruments (400-600 MHz)
- Internal standard methodology
- LA/GA ratio determination
- End-group identification
- Sequence distribution analysis
- Residual monomer quantification
Thermal Analysis:
- Differential Scanning Calorimetry (DSC)
- Modulated DSC for complex transitions
- Glass transition temperature (Tg)
- Melting temperature (Tm)
- Crystallinity percentage
- Heat/cool/heat protocols
- Thermogravimetric Analysis (TGA)
- Thermal stability assessment
- Moisture content determination
- Degradation temperature profiles
End-Group Analysis:
- Organic phase titration for acid number
- NMR spectroscopy for end-group identification
- Correlation with molecular weight data
- Impact assessment on drug-polymer interactions
Drug Content Determination
Multiple Orthogonal Methods:
- Single extraction method (Method 1)
- Multiple extraction protocol (Method 2)
- Amino acid analysis (Method 3)
- UPLC/HPLC with UV detection
- Method selection based on drug-polymer interactions
Excipient Characterization
Gelatin Analysis:
- Ion exchange chromatography for type identification
- GPC for molecular weight distribution
- Bloom number correlation studies
- Amino acid analysis for quantification
- Specific amino acid selection (alanine for gelatin)
Mannitol and Other Excipients:
- Colorimetric assays (enzymatic methods)
- HPLC/UPLC methods
- Gravimetric analysis
- Accurate quantification matching labeled amounts
Selecting the correct polymer grade is essential for regulatory acceptance. Guidance on commonly used grades is provided in PLGA 50:50 supplier considerations: https://resolvemass.ca/plga-5050-supplier/
Alternative biodegradable polymers used in controlled-release systems include PLA and PCL:
- PLA excipient supplier guide: https://resolvemass.ca/pla-excipient-supplier/
- PCL excipient supplier overview: https://resolvemass.ca/pcl-excipient-supplier/
Product Quality Attributes
Particle Characterization:
- Laser diffraction (Mastersizer technology)
- Dynamic light scattering
- Scanning electron microscopy (SEM)
- Surface morphology
- Cross-sectional analysis
- Porosity assessment
- Optical microscopy
Residual Analysis:
- Karl Fischer titration (moisture)
- Gas chromatography (residual solvents)
- Headspace GC methods
- Liquid injection GC methods
- Method optimization for different matrices
- ICH Q3C compliance testing
Release Testing:
- USP dissolution apparatus
- Sample-and-separate methodology
- Temperature-controlled incubation
- Automated sampling systems
- UPLC/HPLC analysis of released drug
- Release profile modeling (f₂ similarity factor)
Regulatory Support Services
ResolveMass Laboratories Inc. provides comprehensive regulatory support for reverse engineering of PLGA polymer in Lupron Depot projects:
Method Development and Validation:
- ICH Q2(R1) compliant validation protocols
- Specificity, linearity, accuracy, precision studies
- Range, LOD, LOQ determination
- Robustness and ruggedness testing
- Transfer protocols for manufacturing sites
- Stability-indicating method development
Specification Setting:
- Statistical analysis of multi-lot RLD data
- Justification of acceptance criteria
- Risk-based specification strategies
- Correlation with clinical performance
Technical Writing:
- Analytical method descriptions
- Validation reports
- Comparative analysis summaries
- DMF (Drug Master File) preparation support
- ANDA module preparation (3.2.P.5)
Regulatory Strategy:
- FDA guidance interpretation
- Q1/Q2 sameness assessment
- Gap analysis vs. RLD
- Deficiency response support
Why ResolveMass for Reverse Engineering of PLGA Polymer in Lupron Depot?
1. Specialized PLGA Expertise
Our team has extensive experience with biodegradable polymer characterization:
- Deep understanding of PLGA chemistry and degradation
- Knowledge of drug-polymer interactions
- Familiarity with microsphere manufacturing processes
- Experience with complex LAI formulations
2. Comprehensive Analytical Platform
State-of-the-art instrumentation specifically calibrated for polymer work:
- GPC systems with multiple detector options
- High-field NMR spectrometers
- Thermal analysis suite (DSC, TGA, DMA)
- Advanced microscopy capabilities
- Chromatography systems (HPLC, UPLC, GC, IC)
3. Regulatory Intelligence
We stay current with evolving FDA expectations:
- Generic LAI guidance documents
- ANDA approval trends and patterns
- Common deficiency responses
- Best practices from successful submissions
4. Quality Systems
ISO-certified laboratory with robust QC:
- Equipment qualification (IQ/OQ/PQ)
- Regular calibration and maintenance
- Method validation expertise
- Data integrity compliance
- Electronic laboratory notebooks
5. Flexible Engagement Models
Customized service packages:
- Single test orders
- Complete characterization panels
- Ongoing project support
- Method transfer and training
- Expert witness services
6. Proven Track Record
Successful support of numerous ANDA submissions:
- Multiple approved generic LAI products
- Complex formulation reverse engineering
- Regulatory milestone achievement
- Client testimonials and references available
7: Best Practices for Reverse Engineering of PLGA Polymer in Lupron Depot
Success requires protecting PLGA from moisture-induced degradation throughout analysis, employing multiple orthogonal analytical methods for parameter verification, analyzing 3-5 RLD batches for statistical validity, maintaining ice-cold conditions during extractions, and correlating all polymer properties with in vitro release performance.
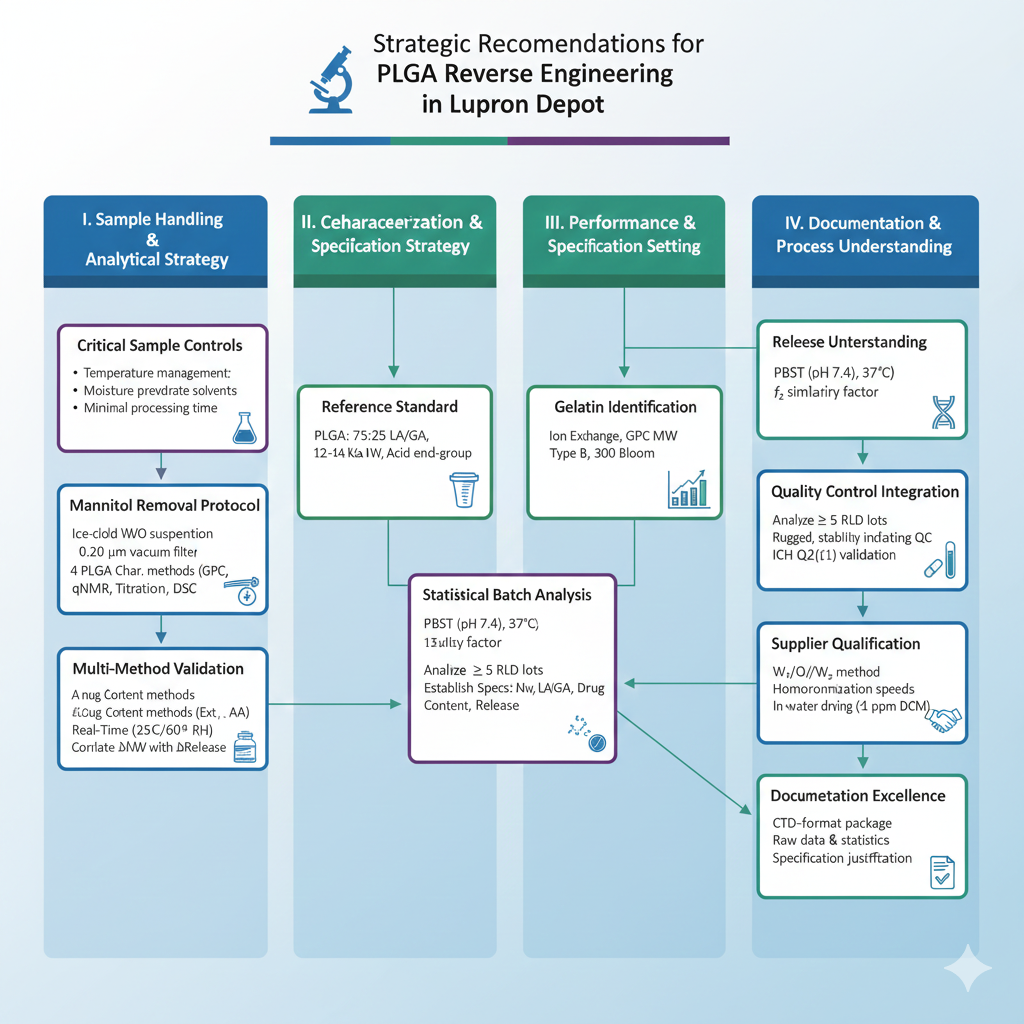
Strategic Recommendations Based on Research Findings
1. Sample Handling and Preparation
Critical Controls:
- Temperature management: Use ice-cold water for initial mannitol removal
- Moisture prevention: Dehydrate all solvents with molecular sieves
- Minimal processing time: Complete extractions quickly to prevent degradation
- Proper storage: Vacuum-dry samples completely, protect from humidity
- Immediate analysis: Analyze GPC samples promptly after preparation
Mannitol Removal Protocol:
- Suspend in ice-cold water (prevents PLGA hydrolysis)
- Vacuum filter through 0.20 μm nylon membrane
- Wash with additional cold water
- Vacuum dry to constant weight
- Verify complete removal by lack of mannitol peaks
2. Multi-Method Validation Strategy
Drug Content Determination: Use three complementary methods:
- Method 1: Single extraction (originator method) – baseline
- Method 2: Multiple extraction (11 cycles) – accounts for drug-polymer binding
- Method 3: Amino acid analysis – independent verification
- Statistical comparison ensures accuracy (all methods p > 0.05 vs. labeled)
PLGA Characterization:
- GPC for molecular weight (Mw, Mn, PDI)
- qNMR for LA/GA ratio and PLGA content
- Titration for acid number (end-group verification)
- DSC for thermal properties
- Cross-validate findings across methods
3. Reference Standard Selection
Gelatin Identification:
- Test multiple commercial gelatin grades
- Perform ion exchange chromatography (type identification)
- Conduct GPC molecular weight distribution analysis
- Create reference microspheres with candidate gelatins
- Extract and compare to RLD gelatin
- Consider regulatory status (low endotoxin, injectable grade)
PLGA Polymer:
- Source PLGA with similar LA/GA ratio (75:25)
- Match molecular weight range (12-14 kDa)
- Verify acid end-group (not ester-capped)
- Obtain Certificate of Analysis from supplier
- Request DMF support file availability
4. Statistical Batch Analysis
Multi-Lot Testing Protocol:
- Analyze minimum 3 lots (preferably 5) of RLD
- Select lots from different manufacturing dates
- Document all parameters for each lot:
- Molecular weight (Mw, Mn, PDI)
- LA/GA ratio
- Drug content
- Gelatin content and MW distribution
- Particle size distribution
- Release kinetics
Statistical Analysis:
- Calculate mean, standard deviation, %RSD
- Establish specification ranges
- Use one-sample t-tests vs. labeled values
- Set generic specifications based on RLD variability
- Document rationale for acceptance criteria
5. Release Testing Optimization
Method Development:
- Medium: PBST (phosphate-buffered saline + 0.02% Tween 80 + 0.02% NaN₃)
- pH: 7.4 (physiological pH)
- Temperature: 37°C incubation
- Agitation: Gentle shaking (avoid excessive mechanical stress)
- Sampling: Sample-and-separate method
- Timepoints: Days 1, 3, 7, then weekly through complete release
- Sample preparation: Centrifugation at 8000 rpm for 5 min
- Complete medium replacement: Fresh PBST at each timepoint
Expected Profile:
- Initial burst: ~23% (Day 1)
- Lag phase: Days 3-30 (slow diffusion)
- Zero-order release: After Day 3
- t₅₀: ~12 days
- Complete release: ~49 days (7 weeks)
Comparative Analysis:
- f₂ similarity factor for profile comparison
- Statistical comparison of key timepoints
- Burst release matching
- Overall release duration equivalence
6. Quality Control Integration
Early QC Method Development:
- Develop rugged methods suitable for routine testing
- Consider automation potential
- Optimize for sample throughput
- Validate methods per ICH Q2(R1)
- Create stability-indicating methods
Transfer Readiness:
- Document method parameters clearly
- Identify critical method variables
- Prepare comprehensive SOPs
- Conduct pre-transfer validation studies
- Plan technology transfer with receiving sites
7. Degradation Study Design
Accelerated Studies:
- Store at 40°C/75% RH (ICH accelerated conditions)
- Monitor multiple parameters simultaneously:
- Molecular weight (Mw, Mn, PDI)
- Drug content and impurities
- Physical appearance
- Particle size distribution
- Release profile changes
- Sampling timepoints: 0, 1, 3, 6 months
Real-Time Studies:
- Store at 25°C/60% RH (ICH long-term conditions)
- Extended timepoints through proposed shelf life
- Correlate polymer degradation with release changes
- Establish stability-indicating specifications
Correlation Analysis:
- Plot Mw vs. time
- Correlate Mw changes with release profile shifts
- Identify critical Mw below which performance fails
- Establish end-of-shelf-life specifications
8. Supplier Qualification Strategy
PLGA Supplier Selection:
- Identify manufacturers with FDA DMF support
- Request regulatory support files
- Verify consistent manufacturing process
- Obtain multiple lot Certificate of Analysis
- Assess batch-to-batch variability
Quality Agreements:
- Define critical polymer specifications
- Establish notification procedures for process changes
- Request annual product reviews
- Maintain qualified supplier list
- Plan for second-source qualification
9. Process Understanding Through Reverse Engineering
Manufacturing Insights:
- Double emulsion (W₁/O/W₂) solvent evaporation method
- Critical process parameters identified:
- Homogenization speeds (15,000 and 12,000 rpm)
- Emulsion cooling to 18°C (viscosity increase)
- Solvent evaporation time (3 hours)
- In-water drying capability (<1 ppm residual DCM)
- These inform generic process development
Formulation Variables:
- PLGA concentration in DCM
- Drug:gelatin ratio in inner aqueous phase
- PVA concentration in outer aqueous phase
- Microsphere washing requirements
- Lyophilization cycle design
10. Documentation Excellence
Comprehensive Reporting:
- Include all raw data and chromatograms
- Document method development rationale
- Provide statistical analysis summaries
- Cross-reference to RLD literature
- Maintain audit trail for all testing
Regulatory Package Preparation:
- Organize data by CTD format (Module 3)
- Prepare comparative analysis tables
- Include representative images (SEM, chromatograms)
- Document specification justifications
- Address potential deficiency questions proactively
8: Advanced Topics in Reverse Engineering of PLGA Polymer in Lupron Depot
Advanced considerations include understanding peptide-polymer electrostatic interactions affecting burst release, microclimate pH effects on stability, the role of gelatin viscosity in manufacturing vs. final product performance, and correlation of initial polymer properties with long-term in vivo release kinetics for IVIVC development.
Understanding Drug-Polymer Interactions
Electrostatic Binding Mechanism:
The reverse engineering of PLGA polymer in Lupron Depot revealed important drug-polymer interactions:
- Cationic leuprolide binds to anionic PLGA carboxyl end-groups
- Ion exchange occurs: Acetate counterion displaced by polymer carboxylate
- Impact on extraction: Single extraction insufficient (8.31% vs. 8.95% with multiple extractions)
- Effect on release: Modulates initial burst and sustained release phases
- Implication: Drug-polymer interactions must be considered in formulation design
Acid Number Significance:
- Acid number 12.9 mg KOH/g indicates abundant carboxylic acid end-groups
- Higher acid number = stronger drug-polymer binding
- Affects microclimate pH during degradation
- Influences peptide stability within microspheres
- Critical specification for generic equivalence
Microclimate pH Considerations
PLGA Degradation Chemistry:
During degradation, PLGA produces acidic byproducts:
- Lactic acid: pKa ~3.86
- Glycolic acid: pKa ~3.83
- Accumulation within microsphere core
- Autocatalytic effect accelerates degradation
Peptide Stability Concerns:
- Low microclimate pH can cause peptide degradation
- Acylation reactions between drug and polymer
- Aggregation and precipitation
- Mitigation strategy: Gelatin may act as pH buffer
Gelatin’s Multiple Roles
Historical vs. Functional:
Original purpose: Increase encapsulation efficiency
- Initial hypothesis: Gelatin viscosity prevented drug loss
- Key discovery: Cooling emulsion increases viscosity (actual mechanism)
- Gelatin retained for consistency with approved formulation
Potential Functions in Final Product:
- Microclimate pH buffering
- Prevention of drug-polymer acylation
- Enhancement of microsphere mechanical properties
- Contribution to controlled release mechanism
- Biocompatibility and safety
Characterization Challenges:
- Gelatin degrades during microsphere manufacturing
- MW distribution shifts to lower values
- Type B 300 bloom pre-manufacturing → degraded profile post-manufacturing
- Must characterize both starting material and extracted gelatin
Particle Size Impact on Performance
Observed Distribution:
- Median diameter: 11.4 μm (narrow distribution)
- 90% below 30 μm (d₀.₉)
- Small, uniform microspheres advantages:
- Uniform drug distribution
- Predictable surface area for release
- Good syringeability
- Reduced inflammation at injection site
Size-Release Correlation:
- Smaller particles: Higher surface area-to-volume ratio
- Expected faster release from smaller particles
- Narrow size distribution: More consistent release
- Critical attribute for bioequivalence
Formulation strategies specific to depot injectables are reviewed in PLGA depot formulation: https://resolvemass.ca/plga-depot-formulation/
Residual Solvent Excellence
Remarkable Achievement:
- Measured: <1 ppm methylene chloride
- Literature value: <100 ppm acceptable
- 100-fold better than expected
Process Implications:
- In-water drying highly effective
- Extended drying time in manufacturing
- Careful control of drying conditions
- Quality by Design approach evident
- Generic manufacturers must match this performance
Regulatory Significance:
- ICH Q3C Class 2 solvent limit: 600 ppm
- LD achieves >600-fold below ICH limit
- Demonstrates pharmaceutical excellence
- Sets high bar for generic equivalence
Release Mechanism Insights
Triphasic Release Pattern:
- Initial Burst (Day 1): 22.8%
- Surface-associated drug release
- Rapid diffusion through water-filled pores
- Drug near microsphere surface
- Controlled by particle size and surface drug distribution
- Lag Phase (Days 3-30): Slow Release
- Diffusion through intact PLGA matrix
- Low water penetration
- Minimal polymer degradation
- Controlled by polymer Mw and crystallinity
- Terminal Release (Days 30-49): Accelerated
- Polymer degradation creates water channels
- Bulk erosion begins
- Autocatalytic effect
- Complete release by Day 49
Zero-Order Kinetics:
- Linear release after Day 3
- Indicates erosion-controlled mechanism
- Therapeutically desirable (constant blood levels)
- t₅₀ = 12.3 days (rapid achievement of therapeutic levels)
Formulation-Specific Considerations
1-Month vs. 3-Month vs. 6-Month Formulations:
Different durations achieved through:
- Molecular weight variation: Higher Mw = slower degradation
- LA/GA ratio adjustment: Higher LA content = more hydrophobic = slower water uptake
- Particle size: Larger particles = longer diffusion path
- Drug loading: Can affect release rate
Reverse Engineering Challenges:
- Each formulation requires separate characterization
- Different PLGA grades used
- Potentially different gelatin or processing
- Independent bioequivalence studies required
9: Future Trends in PLGA-Based LAI Development
Emerging trends include continuous manufacturing processes replacing batch production, advanced PLGA derivatives with enhanced tunability, sophisticated in silico modeling for formulation prediction, patient-centric design approaches, and next-generation depot systems combining multiple drugs or incorporating smart release triggers.
Emerging Technologies
Continuous Manufacturing:
- Replaces batch solvent evaporation
- Quality by Design (QbD) integration
- Process Analytical Technology (PAT)
- Real-time release testing (RTRT)
- Reduced manufacturing time and cost
- Enhanced consistency and control
Advanced PLGA Modifications:
- End-group functionalization: Targeted delivery moieties
- Block copolymers: Enhanced release control
- Branched architectures: Novel degradation profiles
- Surface-modified microspheres: Reduced burst release
- Nanoparticle-in-microsphere: Multimodal release
Regulatory Evolution
FDA Initiatives:
- Enhanced generic LAI guidance documents
- IVIVC development expectations
- Modeling and simulation acceptance
- Risk-based regulatory approaches
- Post-approval change protocols
Patient-Centric Endpoints:
- Injection site reactions
- Pain perception studies
- Ease of administration
- Patient preference studies
- Quality of life assessments
Computational Approaches
In Silico Modeling:
- Polymer degradation prediction
- Release profile simulation
- IVIVC correlation building
- Formulation optimization
- Stability prediction
Artificial Intelligence:
- Machine learning for formulation prediction
- Pattern recognition in analytical data
- Quality attribute prediction
- Process optimization
- Accelerated development timelines
Market Opportunities
Generic Development:
- Multiple LAI products off-patent or nearing expiration
- Significant market sizes justify development investment
- Regulatory pathway clarifying
- Analytical capabilities advancing
Biosimilar Depot Products:
- Long-acting protein and peptide therapeutics
- Complex characterization requirements
- Combination products (drug + device)
- Novel delivery mechanisms
New Chemical Entities:
- Applying LAI technology to new drugs
- Lifecycle management of existing products
- Improved patient compliance
- Competitive differentiation
Conclusion:
Reverse engineering of PLGA polymer in Lupron Depot represents a scientifically sophisticated endeavor that combines advanced analytical chemistry, polymer science expertise, pharmaceutical development knowledge, and regulatory intelligence. The comprehensive characterization described in this case study—from molecular weight determination (Mw ~13.0 kDa, Mn ~8.7 kDa) and compositional analysis (74.3:25.7 LA/GA ratio) to gelatin identification (Type B, 300 bloom) and product attributes (11.4 μm median size, <1 ppm residual solvent)—demonstrates the rigorous approach required for successful generic LAI development.
The success of reverse engineering of PLGA polymer in Lupron Depot hinges on several critical factors: protecting PLGA from degradation throughout analysis, employing orthogonal analytical methods for verification, understanding drug-polymer interactions, characterizing complex excipients like gelatin, and correlating all findings with in vitro release performance. The remarkable quality of the Lupron Depot formulation—evidenced by residual solvent levels 100-fold below literature values and carefully controlled release kinetics—establishes a high benchmark that generic developers must meet.
As demonstrated throughout this detailed case study, the reverse engineering of PLGA polymer in Lupron Depot requires partnership with analytical laboratories possessing specialized capabilities, validated methodologies, and deep regulatory understanding. Organizations investing in comprehensive polymer characterization, multi-lot batch analysis, and correlation with clinical performance position themselves optimally for successful ANDA approval and market entry into this substantial commercial opportunity.
FAQs on PLGA polymer in Lupron Depot:
Reverse engineering PLGA in Lupron Depot involves systematic characterization of the polymer used in the depot formulation to understand its molecular weight distribution, lactide:glycolide (L:G) ratio, end-group chemistry, crystallinity, and degradation behavior. These parameters directly control drug release kinetics in long-acting injectables. Advanced analytical techniques such as GPC/SEC, NMR, DSC, FTIR, and LC-MS are commonly used to recreate a functionally equivalent polymer for generic or reformulated LAI products.
PLGA properties dictate microsphere degradation, water uptake, and erosion rate, which ultimately control drug release over weeks or months. Even minor changes in molecular weight or L:G ratio can significantly alter burst release, dose dumping risk, and therapeutic performance. Therefore, precise PLGA characterization is essential to match the reference listed drug (RLD) behavior and ensure bioequivalence.
Key PLGA attributes include molecular weight (Mn/Mw), polydispersity index (PDI), lactide-to-glycolide ratio, polymer end-group (acid- or ester-terminated), and polymer architecture. Acid-terminated PLGA typically degrades faster, while higher lactide content increases hydrophobicity and slows degradation. These factors together govern the sustained release profile of leuprolide acetate in Lupron Depot.
Common techniques include:
-GPC/SEC for molecular weight and PDI
-¹H & ¹³C NMR for L:G ratio and end-group analysis
-DSC for glass transition temperature (Tg)
-FTIR for functional group confirmation
-LC-HRMS for degradation product profiling
Combining orthogonal techniques ensures accurate polymer fingerprinting.
The L:G ratio is typically determined using proton NMR by integrating characteristic lactide and glycolide peaks. This ratio is crucial because it directly affects polymer hydrophobicity, degradation rate, and drug release duration. Matching the L:G ratio is a regulatory expectation for generic LAI development.
Challenges include polymer extraction without degradation, interference from residual drug or excipients, overlapping degradation products, and batch-to-batch variability. Additionally, proprietary processing methods used by innovators can affect polymer morphology, making functional equivalence more complex than chemical equivalence alone.
Reference
- Hirenkumar K Makadia , Steven J Siegel.Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier.https://pmc.ncbi.nlm.nih.gov/articles/PMC3347861/
- Keiji Hirota, Jia Zhou1, Rose Ackermann, Yan Wang, Stephanie Choi,AnnaSchwendeman and Steven P. Schwendeman.Reverse engineering of the one-month Lupron Depot. https://www.complexgenerics.org/wp-content/uploads/crcg/post-Hirota20161113-AAPS.pdf
- PLGA – A versatile copolymer for design and development of nanoparticles for drug delivery.https://medcraveonline.com/JAPLR/JAPLR-12-00426.pdf

