Summary: Why Benzene-d6 Improves Stability in OLED
- Benzene-d6 enhances OLED stability by replacing C–H bonds with stronger C–D bonds, reducing bond cleavage under electrical stress.
- Deuteration lowers vibrational energy, suppressing exciton-induced molecular degradation pathways.
- Reduced non-radiative decay and lower polaron-induced bond breaking extend device operational lifetime (LT50, LT95).
- Improved thermal and morphological stability minimizes crystallization and phase separation in emissive layers.
- Higher resistance to exciton-polaron annihilation (EPA) results in improved luminance stability.
- Deuterated benzene-d6 derivatives demonstrate superior chemical robustness under high current density and prolonged operation.
- Enables longer device lifetimes without compromising efficiency, especially in blue OLED emitters.
Introduction: Why Benzene-d6 Improves Stability in OLED
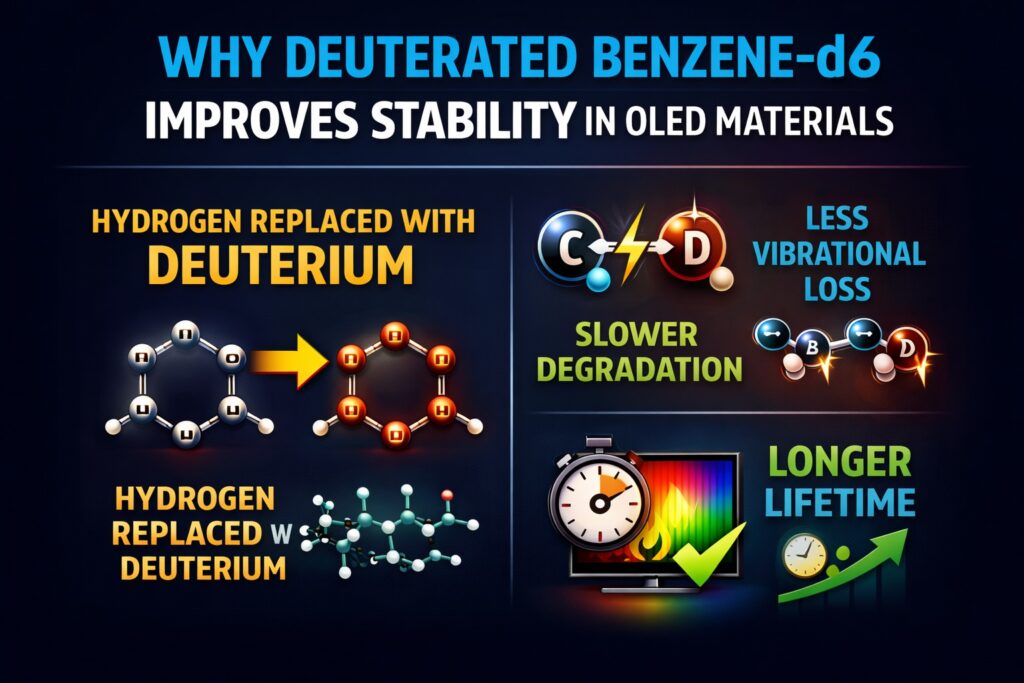
Why Benzene-d6 Improves Stability in OLED is mainly explained by the kinetic isotope effect (KIE). When hydrogen atoms are replaced with deuterium, the C–D bond becomes stronger than the C–H bond. This change slows down chemical reactions that normally cause degradation inside OLED materials. Importantly, this substitution improves stability without significantly changing the electronic structure.
In modern OLED devices, especially high-brightness and blue-emitting systems, degradation often starts at weak C–H bonds. Deuterated benzene-d6 (C₆D₆) forms stronger C–D bonds with lower vibrational energy and slightly higher bond dissociation energy. These features increase resistance to electrical and thermal stress during operation. Even small improvements at the molecular level can make a large difference over thousands of operating hours.
Explore High-Quality Materials: Buy Deuterated Benzene-d6 for OLED Applications
This article explains the molecular mechanisms behind this stability improvement. It also describes how deuteration reduces degradation pathways and improves real device performance. Understanding Why Benzene-d6 Improves Stability in OLED helps manufacturers design longer-lasting and more reliable OLED materials.
Why Benzene-d6 Improves Stability in OLED: Molecular-Level Mechanisms
1. Stronger C–D Bonds Suppress Bond Cleavage
The primary reason Why Benzene-d6 Improves Stability in OLED is the higher bond dissociation energy (BDE) of C–D compared to C–H bonds. Even though the difference is small, it becomes very important during long-term device operation. OLED degradation often begins with repeated microscopic bond-breaking events. Stronger bonds slow this process significantly.
| Bond Type | Approximate Bond Dissociation Energy | Stability Impact |
|---|---|---|
| C–H | ~413 kJ/mol | More susceptible to cleavage under exciton stress |
| C–D | ~418–420 kJ/mol | Greater resistance to bond breaking |
OLED materials are exposed to:
- High exciton density
- Polaron bombardment
- Joule heating
- Exciton-polaron annihilation
Because C–D bonds are stronger, radical formation is reduced. Radicals often trigger chain reactions that damage the emissive layer. By limiting these reactions, deuterated benzene-d6 improves chemical durability and extends device lifetime.
Technical Insight: Detailed Guide on Deuterated Benzene-d6 for OLED
2. Reduced Vibrational Energy Lowers Non-Radiative Decay
Another important reason Why Benzene-d6 Improves Stability in OLED is reduced vibrational energy. Deuterium is heavier than hydrogen, so C–D bonds vibrate more slowly than C–H bonds. Lower vibration means lower zero-point energy inside the molecule. This makes bond breaking less likely under excited conditions.
This effect helps in several ways:
- Reduced internal energy loss
- Suppressed non-radiative decay
- Improved photoluminescence stability
In blue OLED systems, excitons carry high energy. These high-energy states can damage weak bonds. Deuteration reduces vibrational coupling, which protects the molecular structure during prolonged operation.
Partner with Experts: Leading Deuterated Labelled Synthesis Company in Canada
Why Benzene-d6 Improves Stability in OLED Under Exciton Stress
3. Suppression of Exciton-Induced Degradation
Excitons are necessary for light emission, but they can also damage materials. Why Benzene-d6 Improves Stability in OLED becomes clear when we examine exciton-induced bond cleavage. High-energy excitons can break weak molecular bonds and create reactive radicals. These radicals spread degradation through the material.
Deuterated benzene-d6 derivatives show:
- Lower probability of C–D bond rupture
- Reduced radical chain reactions
- Greater chemical durability
This is especially valuable in:
- TADF materials
- Phosphorescent emitters
- High-brightness OLED panels
By slowing exciton-driven damage, deuteration improves both emission stability and overall device lifetime.
Custom Solutions: Request Custom Deuterated Compounds for Your Research
4. Reduced Exciton-Polaron Annihilation (EPA) Damage
Exciton-polaron annihilation creates localized high-energy stress inside OLED layers. These events can break weak bonds and accelerate degradation. Replacing C–H with C–D strengthens the molecular backbone. This reduces the rate of irreversible bond breakage.
As a result:
- Fewer degradation hot spots form
- Radical generation slows down
- Charge transport layers remain stable longer
These improvements directly increase LT50 and LT95 lifetimes. Devices show slower brightness decay and more consistent performance over time.
Quality Assurance: Analytical Characterization of Deuterated Compounds
Why Benzene-d6 Improves Stability in OLED: Thermal and Morphological Factors
5. Enhanced Thermal Stability
OLED fabrication and operation involve continuous heating. Materials must survive vacuum deposition, annealing, and Joule heating. Deuterated benzene-d6 shows modest but meaningful improvement in thermal resistance. Stronger bonds resist thermal cleavage and structural rearrangement.
Even small thermal improvements reduce long-term degradation. Stable materials maintain chemical integrity during manufacturing. This supports better reproducibility and long-term reliability in OLED devices.
Industrial Supply: Trusted Supplier of Deuterated Reagents
6. Improved Morphological Stability
Morphological instability, such as crystallization or phase separation, leads to luminance decay. Deuterated benzene-d6 supports more stable molecular packing. Thin films remain uniform over extended operating periods. This prevents microstructural defects that reduce performance.
Key benefits include:
- Reduced microphase separation
- Better thin-film uniformity
- Increased stability in host-dopant systems
Maintaining film structure is critical for blue emitters and high-brightness displays. Stable morphology ensures consistent emission characteristics.
Why Benzene-d6 Improves Stability in OLED Blue Emitters
Blue OLEDs operate at higher energy levels, which increases degradation risk. High-energy excitons attack the weakest bonds first. By replacing C–H with C–D, vulnerable sites become stronger. This directly improves resistance to fragmentation.
Why Benzene-d6 Improves Stability in OLED blue systems is strongly linked to this selective bond reinforcement.
Observed benefits include:
- Delayed molecular breakdown
- Lower luminance decay
- Extended LT95 lifetime
- Improved photochemical stability
Many studies report:
- 1.5×–3× longer operational lifetime
- Similar or slightly improved efficiency
- Minimal shift in emission spectrum
These results make deuteration highly valuable for blue OLED durability.
Browse Our Catalog: Availability of All Deuterated Chemicals
Why Benzene-d6 Improves Stability in OLED: Device-Level Impact
7. Extended Operational Lifetime (LT50, LT95)
Device-level testing clearly shows the impact of deuteration. By slowing chemical breakdown, brightness declines more gradually. Dark spot formation is also reduced. This improves overall display reliability.
Common improvements include:
- Longer LT50 at 1000 cd/m²
- Reduced efficiency roll-off
- Improved stability at high current density
These benefits are critical for commercial OLED applications in displays and lighting.
Precision Benchmarking: View Deuterated Internal Standards
8. Maintained Quantum Efficiency
Importantly, deuteration does not significantly change electronic energy levels. The substitution affects atomic mass, not electron distribution. This means charge mobility and energy alignment remain stable. Performance gains come without sacrificing efficiency.
OLED materials containing benzene-d6 typically show:
- Stable HOMO/LUMO alignment
- Comparable charge transport
- Minimal emission wavelength change
This balance between stability and efficiency explains Why Benzene-d6 Improves Stability in OLED without negative side effects.
Practical Considerations for Using Benzene-d6 in OLED Materials
When incorporating benzene-d6, careful design is required. The degree of deuteration and its position in the molecule influence stability. Full deuteration provides maximum reinforcement, while selective deuteration targets weak sites. Cost and scalability must also be considered.
Consider:
- Required degree of deuteration
- Position-specific versus full substitution
- Synthetic scalability
- Cost-performance balance
Applications:
- Host materials
- TADF emitters
- Blue phosphorescent emitters
- Hole transport materials
High-purity benzene-d6 is essential to avoid isotopic dilution. Controlled synthesis ensures consistent OLED device performance.
Global Sourcing: Deuterated Labelled Chemical Synthesis Company in United States
Conclusion: Why Benzene-d6 Improves Stability in OLED
Why Benzene-d6 Improves Stability in OLED comes down to stronger molecular bonds and reduced vibrational stress. Replacing C–H bonds with C–D bonds slows degradation caused by excitons, heat, and electrical stress. Radical formation decreases, and chemical breakdown is delayed.
These molecular advantages translate into longer device lifetimes, especially in demanding blue OLED systems. Efficiency remains stable while operational hours increase. For manufacturers focused on durability and performance, deuterated benzene-d6 offers a proven and practical stability strategy.
Ready to Start? Buy Deuterated Compounds Online
Frequently Asked Questions (FAQs)
Deuteration strengthens the molecular structure by replacing C–H bonds with stronger C–D bonds. These stronger bonds are less likely to break under electrical stress and high-energy excitons. This slows down chemical degradation inside the emissive layer. As a result, OLED devices can operate for a longer time before noticeable brightness loss occurs.
In most cases, benzene-d6 does not reduce OLED efficiency. The electronic structure of the material remains almost the same because deuteration changes atomic mass, not electron distribution. Charge transport and energy alignment stay stable. This allows the device to maintain strong light output while improving durability.
Benzene-d6 is especially valuable in blue OLED systems because blue emitters operate at higher energy levels. Higher energy increases the risk of bond breaking and faster degradation. By strengthening vulnerable bonds, deuteration helps blue materials last longer. However, it can also benefit other OLED colors.
Deuteration typically does not cause a noticeable change in emission color. The light emission process depends on electronic transitions, which remain mostly unaffected. Since isotopic substitution mainly alters bond vibration, spectral properties stay nearly the same. This means color purity is preserved.
Yes, stronger C–D bonds are more resistant to damage caused by exciton-polaron interactions. These high-energy events can break weaker bonds and create reactive species. By reinforcing the molecular backbone, deuteration reduces structural damage. This contributes to improved operational stability.
Benzene-d6 derivatives are compatible with common OLED fabrication methods such as vacuum deposition. They behave similarly to non-deuterated materials in processing conditions. Maintaining high material purity is essential for consistent device results. Proper handling ensures optimal performance.
Reference
- Di Martino, R. M., Maxwell, B. D., & Pirali, T. (2023). Deuterium in drug discovery: Progress, opportunities and challenges. Nature Reviews Drug Discovery, 22(7), 562–584. https://doi.org/10.1038/s41573-023-00703-8
- Kopf, S., Bourriquen, F., Li, W., … & Morandi, B. (2022). Recent developments for the deuterium and tritium labeling of organic molecules. Chemical Reviews, 122(6), 6634-6713. https://doi.org/10.1021/acs.chemrev.1c00795
- Munir, R., Zahoor, A. F., Khan, S. G., Hussain, S. M., Noreen, R., Mansha, A., Hafeez, F., Irfan, A., & Ahmad, M. (2025, August 21). Total syntheses of deuterated drugs: A comprehensive review. Top Current Chemistry (Cham), 383(3), 31. https://doi.org/10.1007/s41061-025-00515-x