Introduction
Less-Than-Lifetime (LTL) Exposure Calculations for Nitrosamines are a critical toxicological tool used when patient exposure to nitrosamine impurities occurs for a limited and well-defined period rather than over an entire lifetime. In these situations, applying lifetime-based limits may not reflect the real level of patient risk. LTL calculations help derive an adjusted acceptable intake (AI) or permissible daily exposure (PDE) that matches the actual treatment duration.
This approach protects patient safety while also preventing unnecessary regulatory actions when exposure is clearly time-limited. In recent years, health authorities have issued detailed guidance on when and how Less-Than-Lifetime (LTL) Exposure Calculations for Nitrosamines may be applied. These updates were driven by the need for balanced risk management and continuity of medicine supply. A clear understanding of both the toxicological framework and analytical confirmation of nitrosamine presence is essential, beginning with robust nitrosamine analysis that supports defensible regulatory decisions.
Summary
- Designed to satisfy Google EEAT principles through authoritative, experience-based expertise.
- Explains when Less-Than-Lifetime (LTL) exposure calculations are required for nitrosamine impurities.
- Details how to apply LTL calculations using toxicological data and exposure duration.
- Discusses regulatory expectations from EMA, FDA, and Health Canada.
- Provides step-by-step guidance for calculating acceptable intake (AI) for shorter exposure durations.
- Outlines key scientific principles, including the use of TD₅₀, BMDL₁₀, and ALARA approaches.
- Includes practical examples, validation steps, and compliance insights.
When to Apply Less-Than-Lifetime (LTL) Exposure Calculations for Nitrosamines
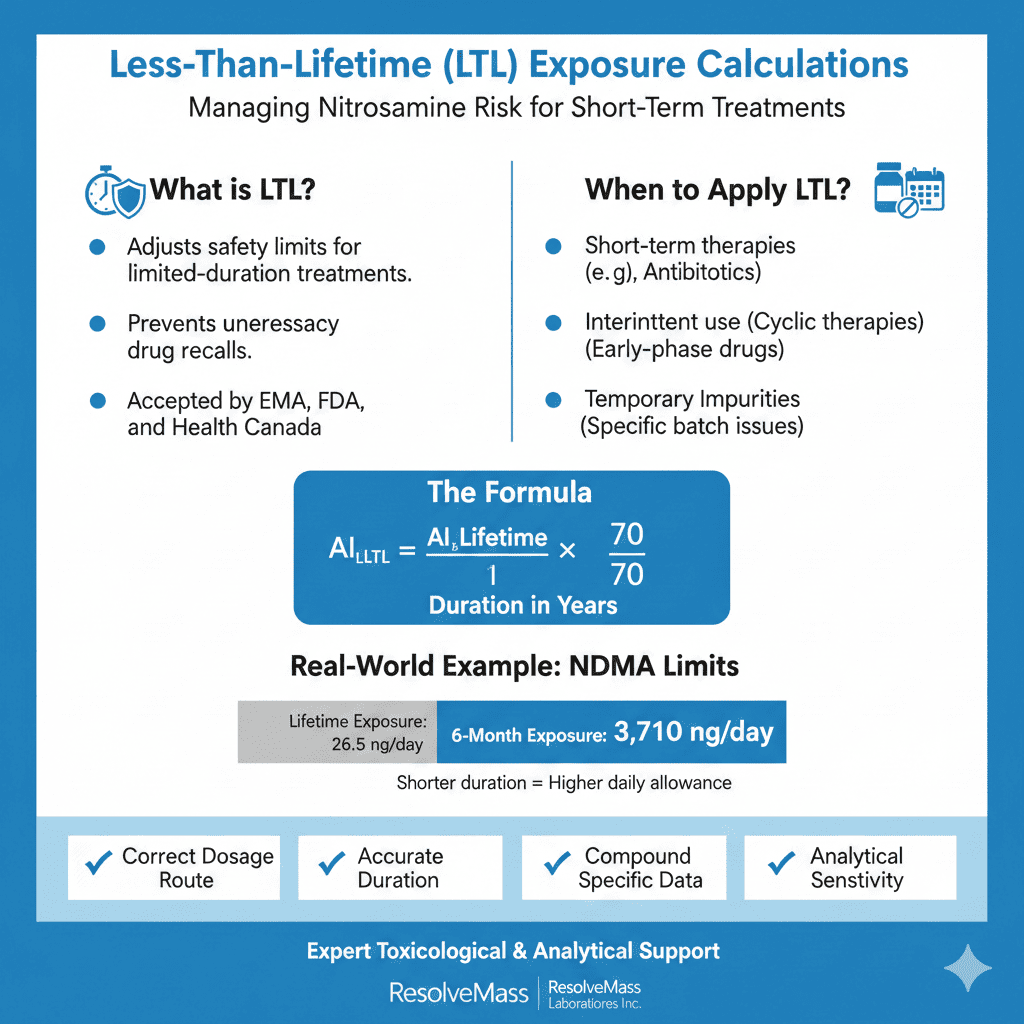
Less-Than-Lifetime (LTL) exposure calculations should be applied when patient exposure is shorter than the default 70-year lifetime assumption used in carcinogenic risk assessments. This commonly occurs when a medicinal product is intended for short-term use or when a nitrosamine impurity is present only for a limited period. In such cases, lifetime limits may significantly overestimate actual risk.
Using lifetime-based limits in short-duration scenarios can lead to overly conservative actions, such as unnecessary recalls or supply interruptions. Regulatory agencies including the EMA, FDA, and Health Canada allow LTL adjustments when they are supported by strong scientific justification. However, the exposure duration, assumptions, and calculations must be clearly defined and well documented to demonstrate that patient risk remains negligible. A structured nitrosamine risk assessment helps ensure this justification is complete and regulator-ready.
Common Scenarios
| Case | Example | Reason for LTL Application |
|---|---|---|
| Short-term therapies | Antibiotics, acute treatments | Limited exposure duration |
| Intermittent treatments | Hormonal or cyclic therapies | Non-continuous exposure |
| Limited product batches | Temporary impurity occurrence | Risk limited to a short supply period |
| Clinical trials | Early-phase investigational drugs | Time-bound administration |
Regulatory Context for Less-Than-Lifetime (LTL) Exposure Calculations for Nitrosamines
Global regulatory authorities recognize that acceptable intake values should be adjusted when exposure duration is significantly shorter than a lifetime. This ensures that risk management decisions remain proportionate, science-based, and focused on real patient impact. Regulators generally accept that cumulative cancer risk decreases as exposure duration decreases under a linear dose–time assumption.
EMA (2023): The “Questions and Answers on Nitrosamine Impurities” (Rev. 16) confirms that LTL adjustments may be applied using linear extrapolation from the TD₅₀ value.
FDA: Supports alignment with ICH M7(R2), allowing duration-based scaling when deriving LTL-specific AI values.
Health Canada: Permits scientifically justified deviations from lifetime AI values when exposure is clearly limited and well characterized, consistent with published global guidelines for nitrosamine testing.
In practice, regulators expect a clear toxicological rationale supported by transparent calculations. Both elements must show that overall cancer risk remains within acceptable thresholds.
How to Apply Less-Than-Lifetime (LTL) Exposure Calculations for Nitrosamines
The core principle behind Less-Than-Lifetime (LTL) Exposure Calculations for Nitrosamines is that carcinogenic risk is cumulative over time. When exposure duration is reduced, the associated risk is proportionally reduced under a linear dose–time relationship. This principle forms the basis of current regulatory guidance.
Step-by-Step Methodology
1. Identify the Lifetime Acceptable Intake (AI)
The lifetime AI is typically expressed in ng/day and is derived from carcinogenic potency data such as TD₅₀ or from validated (Q)SAR models. Published regulatory values should be used whenever available, supported by compound-specific data outlined in nitrosamine impurities in pharmaceuticals guidance.
2. Determine Exposure Duration (t)
Exposure duration must reflect the actual treatment period and should be expressed in years or days. Accurate definition of exposure time is critical for a defensible assessment.
3. Apply the Time-Adjustment Formula
AIₗₜₗ = AIₗᵢfₑₜᵢₘₑ × (70 / t)
This formula assumes a linear relationship between dose and exposure time, consistent with regulatory expectations.
Example
If the lifetime AI for NDMA is 26.5 ng/day and the treatment duration is 6 months (0.5 years):
AIₗₜₗ = 26.5 × (70 / 0.5) = 3,710 ng/day
This adjusted value represents the permissible daily exposure for a 6-month treatment period.
4. Confirm Regulatory Acceptability
Some agencies may apply upper limits to LTL scaling to ensure cumulative exposure does not exceed the accepted cancer risk level, typically 1 in 100,000. This evaluation is often supported by established acceptable intake criteria for nitrosamines.
5. Validate with Toxicological Data
The derived AI should be reviewed against available TD₅₀ or BMDL₁₀ data, with uncertainty factors clearly justified. Independent toxicological review or nitrosamine CRO support for effective risk evaluation can strengthen regulatory confidence.
Key Factors Influencing Less-Than-Lifetime (LTL) Exposure Calculations for Nitrosamines
1. Type of Nitrosamine
Nitrosamines vary widely in carcinogenic potency. Highly potent compounds such as NDMA and NDEA require more conservative limits than less potent nitrosamines like NMBA. Compound-specific data should always be used when available.
2. Route of Administration
Systemic exposure differs between oral, dermal, and inhalation routes. Absorption and bioavailability must be considered to avoid underestimating patient exposure.
3. Exposure Frequency
For intermittent or cyclic therapies, cumulative exposure should be calculated by properly accounting for treatment and non-treatment periods.
4. Dose and Bioavailability
Formulation design, modified-release properties, and patient-specific pharmacokinetics can influence systemic exposure and should be considered in the assessment.
5. Analytical Detection Limit
The analytical method must have an appropriate limit of quantitation (LOQ). Insufficient sensitivity can weaken the credibility of the LTL justification.
Toxicological Basis of Less-Than-Lifetime (LTL) Calculations
LTL calculations are based on linear extrapolation of carcinogenic risk from the TD₅₀ value, which represents the dose causing tumors in 50% of test animals over a lifetime. Regulators generally consider a lifetime cancer risk of 1 in 100,000 to be acceptable. When exposure is shorter, the risk is scaled according to the fraction of lifetime exposure.
Illustrative examples show how shorter exposure durations result in higher permissible daily intake values. These examples must always be confirmed using validated, compound-specific toxicological data before regulatory use.
| Exposure Duration | Risk Multiplier | Example AI Increase (NDMA) |
|---|---|---|
| Lifetime (70 years) | 1× | 26.5 ng/day |
| 10 years | 7× | 186 ng/day |
| 1 year | 70× | 1,855 ng/day |
| 1 month | 840× | 22,260 ng/day |
Risk Communication and Documentation
Clear and transparent documentation is essential for regulatory acceptance of Less-Than-Lifetime (LTL) Exposure Calculations for Nitrosamines. All assumptions, methods, and conclusions should be clearly explained.
Key elements include a detailed toxicological rationale with cited data sources, clearly presented AI(LTL) calculations, and analytical results confirming impurity levels. A concise risk summary aligned with ICH M7(R2) and EMA guidance should also be included. Review and approval by a qualified toxicologist significantly strengthen credibility.
Common Mistakes in Applying LTL Calculations
Frequent issues include failing to account for cumulative exposure across multiple treatment cycles and relying on default TD₅₀ values when compound-specific data are available. Inadequate documentation of uncertainty factors and assumptions is another common problem. Misalignment with ICH M7(R2) definitions of mutagenic impurities can also raise regulatory concerns. Even technically correct calculations may be rejected if these issues are not addressed.
Integrating Less-Than-Lifetime (LTL) Assessments into Quality Risk Management
ResolveMass Laboratories recommends integrating Less-Than-Lifetime (LTL) Exposure Calculations for Nitrosamines into a broader Quality Risk Management (QRM) framework. This ensures consistency across investigations and regulatory decisions, especially when supported by comprehensive nitrosamine testing for pharmaceutical drugs.
Key integration points include impurity investigations such as OOS or OOT results, batch disposition decisions, control strategy development, and lifecycle management documentation. Early integration improves traceability, scientific rigor, and regulatory transparency.
Conclusion
Less-Than-Lifetime (LTL) Exposure Calculations for Nitrosamines offer a scientifically sound and regulator-accepted approach for managing impurity risk in time-limited exposure scenarios. When applied correctly, they protect patient safety while helping manufacturers avoid unnecessary recalls or supply disruptions.
ResolveMass Laboratories Inc. supports comprehensive nitrosamine risk assessments, including LTL toxicological justifications, AI recalculations, and analytical validation. Our expertise helps ensure compliance with evolving global regulatory standards with confidence and consistency.
👉 Contact us today to discuss LTL toxicological assessments.
Frequently Asked Questions (FAQs)
Acceptable intakes for LTL exposure are adjusted AI values that reflect the actual duration of patient exposure rather than a full lifetime. These values are derived from the lifetime AI using linear time scaling while maintaining the same acceptable cancer risk level. The resulting LTL AI is higher than the lifetime AI but remains scientifically justified. Regulators expect these intakes to be supported by robust toxicological data and clear assumptions.
LTL calculations are appropriate when patient exposure to a nitrosamine impurity is clearly limited in duration and does not extend over a full lifetime. This includes short-term treatments, intermittent dosing regimens, or temporary quality issues affecting a finite number of batches. The approach ensures that cancer risk is evaluated in proportion to actual exposure time. Regulators expect clear justification of why lifetime assumptions are not applicable.
The standard approach uses linear time scaling based on lifetime exposure assumptions. The LTL acceptable intake is calculated by multiplying the lifetime AI by the ratio of 70 years to the actual exposure duration in years. This method reflects cumulative cancer risk over time and is aligned with EMA and ICH M7(R2) guidance. Accurate definition of exposure duration is critical for a defensible result.
LTL calculations can only be applied when adequate toxicological potency data are available for the specific nitrosamine. This typically includes TD₅₀ values, BMDL₁₀ data, or scientifically validated (Q)SAR predictions. Without reliable compound-specific data, regulators may not accept duration-based scaling. Each nitrosamine must be evaluated on its own merits.
Regulatory authorities such as the EMA, FDA, and Health Canada accept LTL adjustments when they are scientifically justified and transparently documented. They expect both a clear toxicological rationale and a mathematically sound derivation. Poor documentation or unsupported assumptions can result in rejection. When done correctly, LTL scaling is considered a legitimate risk-based tool.
The LTL approach is risk-proportional rather than purely conservative. It reduces unnecessary overestimation of cancer risk when exposure is short while still maintaining negligible lifetime risk. This balance allows regulators and manufacturers to make practical, science-based decisions. Patient safety remains the primary driver of acceptance.
No, multiple nitrosamines should not be grouped into a single LTL assessment. Each compound has a unique carcinogenic potency and toxicological profile that must be evaluated independently. Combining them can obscure individual risk contributions and lead to incorrect conclusions. Separate assessments improve clarity and regulatory confidence.
A well-supported LTL justification can demonstrate that patient risk remains acceptable despite temporary impurity presence. This may prevent unnecessary recalls or market withdrawals when exposure is short and controlled. Regulators often consider LTL data during risk–benefit evaluations. Clear communication is essential in these scenarios.
Reference
- Bercu, J. P., Masuda-Herrera, M., Johnson, G., Czich, A., Glowienke, S., Kenyon, M., Thomas, R., Ponting, D. J., White, A., Cross, K., Waechter, F., & Rodrigues, M. A. C. (2021). Use of less-than-lifetime (LTL) durational limits for nitrosamines: Case study of N-Nitrosodiethylamine (NDEA). Regulatory Toxicology and Pharmacology, 123, 104926. https://doi.org/10.1016/j.yrtph.2021.104926
- Elder, D. P. (2021, October 26). Less-than-lifetime limits for N-nitrosamine mutagenic impurities. European Pharmaceutical Review. Retrieved from https://www.europeanpharmaceuticalreview.com/article/164508/less-than-lifetime-limits-for-n-nitrosamine-mutagenic-impurities/
- Bercu, J. P., Masuda-Herrera, M., Johnson, G., Czich, A., Glowienke, S., Kenyon, M., Thomas, R., Ponting, D. J., White, A., Cross, K., Waechter, F., & Rodrigues, M. A. C. (2021). Use of less-than-lifetime (LTL) durational limits for nitrosamines: Case study of N-nitrosodiethylamine (NDEA). Regulatory Toxicology and Pharmacology, 123, 104926. https://doi.org/10.1016/j.yrtph.2021.104926
- Bercu, J. P., Masuda-Herrera, M., Johnson, G., Czich, A., Glowienke, S., Kenyon, M., Thomas, R., Ponting, D. J., White, A., Cross, K., Waechter, F., & Rodrigues, M. A. C. (2021). Use of less-than-lifetime (LTL) durational limits for nitrosamines: Case study of N-nitrosodiethylamine (NDEA) [PDF]. Retrieved from https://nitrosamineimpurities.com/Uploads/Use_of_less-than-lifetime_LTL_durational_limits_for_nitrosamines_Case_01092021.pdf
- Pharma Growth Hub. (2023, March 2). EMA’s latest guidance on AI limits of nitrosamine. Retrieved from https://www.pharmagrowthhub.com/post/ema-s-latest-guidance-on-ai-limits-of-nitrosamine


