Peptide characterization has become a critical step for generic drug developers preparing Abbreviated New Drug Applications (ANDA) or other U.S. FDA submissions. Whether you’re working on a generic version of a peptide-based API, a complex modified peptide, or a formulation containing therapeutic peptides, detailed characterization is not just a regulatory requirement for Health Canada and USFDA—it’s essential for demonstrating equivalence, safety, and quality.
With the FDA and Health Canada increasing its focus on structural integrity, impurity profiling, and batch-to-batch consistency in 2025, selecting the right analytical partner can directly influence your project’s approval timeline.
Important Note
Important Notice:
ResolveMass Laboratories works exclusively with pharmaceutical and biotech companies, providing analytical services to support their NDA and ANDA project submissions to FDA and Health Canada for peptides.
We do not offer any testing services to individuals or for personal use. Our services are strictly for business and research purposes only.
Our laboratory operates strictly under a research and development (R&D) framework for the pharma and biotech companies. Testing is intended solely for research purposes to the pharma and biotech companies and is not authorized for clinical or diagnostic decision-making in any forms. For medical use guidance, please consult a licensed healthcare professional.
Why Peptide Characterization Matters for Generic Drug Submissions in the U.S.
For a generic peptide product to gain FDA approval, developers must prove it matches the Reference Listed Drug (RLD) in identity, purity, potency, and structural features. Peptide characterization goes beyond simple purity checks—it includes comprehensive analysis to:
- Confirm the primary sequence and post-translational modifications
- Detect and quantify impurities, including degradation products
- Assess physicochemical properties and stability
- Support CMC and comparability data for regulatory filings
ResolveMass Laboratories Inc., a Canada-based analytical CRO, serves U.S. generic drug manufacturers with advanced peptide characterization services aligned with FDA and ICH requirements.
ResolveMass Laboratories Inc., a Canada-based analytical CRO, serves clients across the U.S. with cutting-edge peptide testing services that meet global quality benchmarks.
Key Aspects of a Reliable Peptide Characterization Lab for U.S. Generic Projects
A dependable analytical partner should offer:
- Regulatory-Ready Methodologies — Validated methods per ICH Q2(R1)
- Comprehensive Impurity Profiling — Detection down to 0.1% as per FDA guidelines
- Rapid Turnaround Times — Minimizing delays in ANDA submission timelines
- CMC Data Support — Documentation suitable for FDA Module 3
- Secure Data Management — 21 CFR Part 11–compliant records
ResolveMass delivers these capabilities, combining analytical expertise with regulatory insight to generate submission-ready data packages.s is discussed in this article: Cost of Peptide Analysis Service: What Affects Pricing and How to Budget
Core Techniques Used in Peptide Characterization for Generic Drug Submissions
Modern labs offering peptide purity testing typically rely on:
✅ 1. High-Performance Liquid Chromatography (HPLC)
- High-performance liquid chromatography (HPLC) is the preferred method for verifying peptide purity; researchers trust it for its accuracy and dependability.
- Reliable, adaptable, and offering a clear chromatographic profile—absolutely necessary for measuring purity before more characterisation.
- What makes HPLC so essential? ✔ Versatility – From hydrophobic to hydrophilic, a broad spectrum of peptides is addressed.
✔ Reproducibility – Consistent results run after run, crucial for QC in synthesis.
✔ Quantitative Accuracy – precise purity calculations are made possible by clear peak integration.
✅ 2. Ultra-Performance Liquid Chromatography (UPLC)
- When speed and sensitivity matter, UPLC (Ultra-Performance Liquid Chromatography) delivers. As HPLC’s advanced counterpart, it uses higher pressures and smaller particles to achieve:
- ✔ Faster runs – Get results in minutes, not hours
- ✔ Sharper peaks – Better separation for tricky impurities
- ✔ Lower solvent use – More eco-friendly workflows
✅ 3. Liquid Chromatography-Mass Spectrometry (LC-MS)
- LC-MS offers both separation and molecular identification making it essential for peptide purity testing. This approach provides researchers with a comprehensive solution for purity assessment, offering several key advantages:
✔ Component Clarity – First, LC separates out each peptide and impurity so you can see exactly what’s in your sample.
✔ Molecular Insight – Then MS measures the precise mass of each compound, helping you confirm if it’s your target peptide or an impurity.
✔ Trace Impurity Detection – Even tiny levels of deletion sequences, truncations, or modified amino acids are revealed—critical for regulatory submissions.
✔ Ideal for Modified Peptides – Need to check phosphorylation or lipidation? LC-MS handles complex post-translational modifications effortlessly.
✔ Sequence Support – Go further with LC-MS/MS to break down the structure and confirm amino acid sequences, ideal for identity verification.
✅ 4. Capillary Electrophoresis (CE)
- Capillary Electrophoresis (CE) is a high-efficiency, electrokinetic separation technique widely used for peptide purity testing, specially for charged or hydrophilic peptide analytes.
✔ Separation Based on Electrophoretic Mobility
It uses a narrow capillary and applies a high voltage to separate peptides based on their charge-to-hydrodynamic size ratio. This silica fused capillary provides a high surface-to-volume ratio, which ensures heat dissipation and fast separations using high voltage.
✔ Resolution of Hydrophilic and Charged Impurities
In many cases, some impurities are less detectable by reversed-phase HPLC. So ,CE is highly effective because it detects hydrophilic impurities, salt forms, degradation products, and polar contaminants. It is especially useful for basic or acidic peptides and peptide drugs with isomeric forms.For peptide-based drugs where subtle charge differences matter, CE provides an edge that HPLC simply can’t.
✔ Low Sample and Solvent Requirements
Capillary Electrophoresis (CE) is incredibly efficient when it comes to sample economy — it typically uses just nanoliter volumes. This makes it especially valuable for rare, expensive, or low-yield peptide batches. Plus, with minimal reagent use, CE supports greener, more sustainable lab practices — a growing priority in modern analytical development.
✅ 5. Mass Spectrometry (MS)
- Mass spectrometry is considered the gold standard for confirming peptide identity and integrity. It offers highly accurate molecular weight determination and in-depth structural insights — making it indispensable in peptide purity testing workflows. Beyond simply verifying mass, MS helps detect post-translational modifications, sequence variants, and low-level impurities that other techniques might miss.
- Whether you’re analyzing synthetic peptides or biologics, MS brings clarity and confidence to your data. Its ability to validate purity and structural accuracy is especially crucial for regulatory submissions, quality assurance, and advanced research applications. In short, if precision and confidence matter — MS is the tool you want backing your results.
✅ 6. Amino Acid Analysis (AAA)
- Breaks Down Peptides to Their Core Components
AAA hydrolyzes the peptide into free amino acids and quantifies each one — giving you a full breakdown of its composition. - Confirms Peptide Identity
By comparing the detected amino acid profile to the theoretical one, you can confirm whether the right sequence was synthesized. - Ensures Composition Accuracy
Ideal for batch-to-batch consistency checks, especially for therapeutic or research-grade peptides. - Detects Synthesis Errors
Missing or extra amino acids due to deletion or insertion errors show up clearly in the amino acid profile. - Supports High-Quality Peptide Purity Testing in the United States
AAA is often part of regulatory-compliant workflows — especially in GMP environments where documentation of composition is required. - Essential for Peptides Used in Biopharma & Diagnostics
For peptides that serve as active ingredients or critical reagents, confirming composition isn’t optional — it’s essential.
✅ 7. Peptide Mapping
- What It Is
Peptide mapping involves enzymatically digesting the peptide (commonly with trypsin) into smaller fragments, which are then analyzed using HPLC-MS (Liquid Chromatography–Mass Spectrometry). - Detects Sequence Errors
If there are misincorporated amino acids, deletion mutations, or truncations, peptide mapping helps pinpoint their exact locations. - Reveals Post-Synthetic Modifications
Whether it’s oxidation, acetylation, deamidation, or disulfide bond mispairing, peptide mapping brings these to light by comparing fragment masses. - Validates Structural Integrity
Essential for confirming that the final peptide product matches the theoretical sequence — especially for therapeutic candidates and research-grade peptides. - Supports Peptide Purity Testing in the United States
Widely accepted by FDA, USP, and GLP/GMP laboratories as a gold-standard method for structural verification. - Complementary to MS and AAA
While MS confirms molecular weight, and AAA checks amino acid composition, peptide mapping shows how the pieces are connected — offering a full structural fingerprint.
Explore Our Peptide Characterization Services
Why Choose ResolveMass Laboratories Inc. for Peptide Characterization service in the United States?
ResolveMass stands out due to its:
- State-of-the-art facilities with validated equipment and software
- Extensive experience in peptide analysis across research, clinical, and GMP contexts
- Customized protocols based on molecular complexity
- Dedicated client support for U.S.-based customers
Explore Our Peptide Sequencing Services
Case Study: Peptide Characterization for a U.S. ANDA Submission
Client: U.S.-based generic manufacturer developing a peptide API equivalent to an RLD.
Objective: Generate a full peptide characterization dossier, including:
- Primary sequence confirmation
- Impurity profiling to <0.1%
- Physicochemical property assessment
Approach: HPLC, LC-MS/MS, CE, and peptide mapping, validated per ICH Q2(R1).
Outcome: Complete CMC Module 3 package delivered in 6 business days, meeting FDA technical requirements. Client successfully filed ANDA with no deficiencies related to peptide analytical data.
Factors to Consider When Selecting a Peptide Characterization Lab in the United States
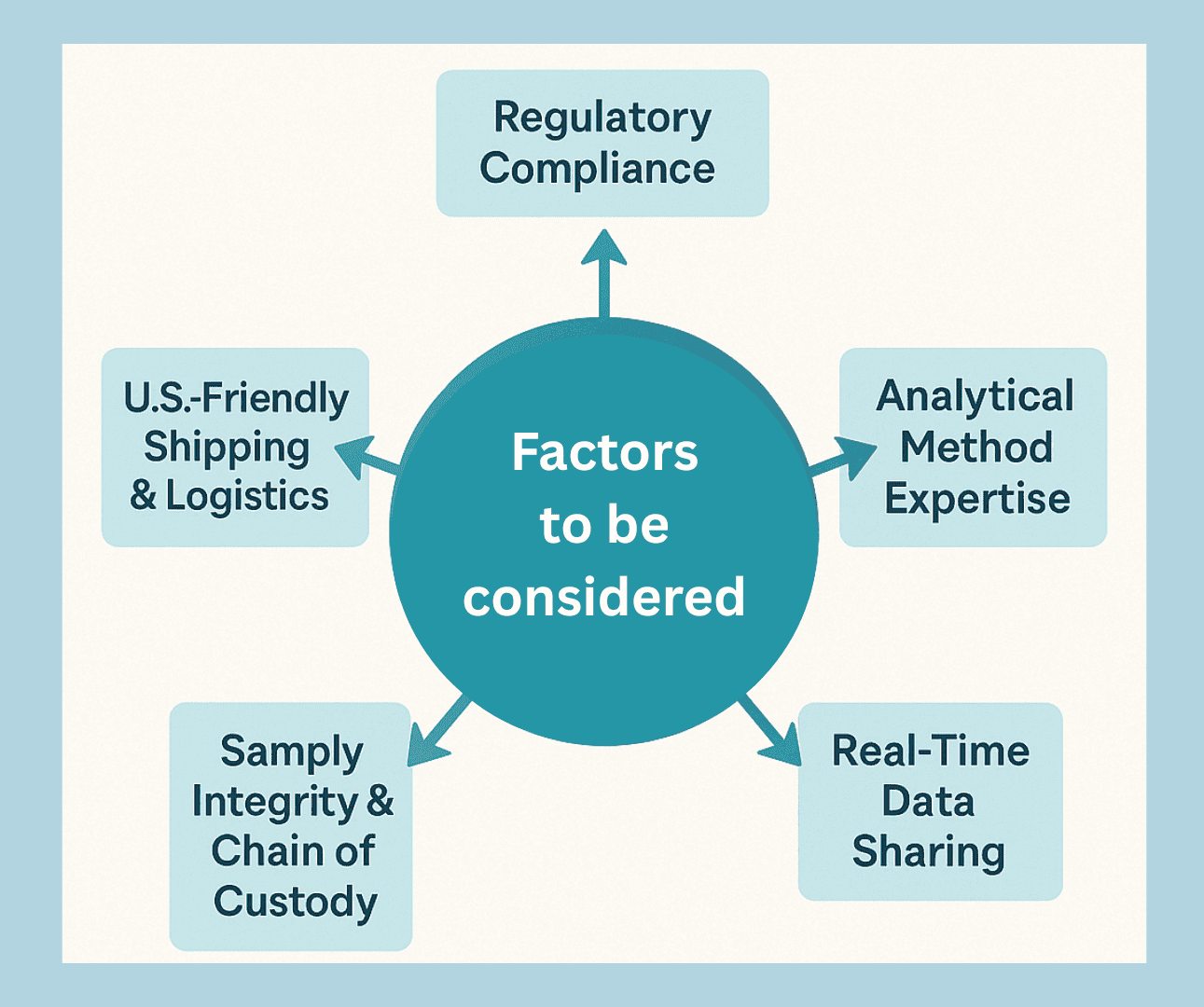
1. Regulatory Compliance
Ensure your lab partner adheres to GLP or GMP standards where required.
2. Analytical Method Expertise
Choose labs that can develop and validate custom methods for your unique peptide structure.
3. Real-Time Data Sharing
Look for partners offering online portals or secure dashboards to access chromatograms and reports.
4. Sample Integrity & Chain of Custody
A reliable peptide characterization lab in the United States must ensure complete traceability and sample safety.
5. U.S.-Friendly Shipping & Logistics
ResolveMass offers streamlined cross-border logistics with expedited customs clearance to serve U.S. clients faster.
Visit Our Peptide Characterization Service Page
Conclusion
For U.S. generic drug submissions involving peptides, characterization is more than a technical exercise—it’s the backbone of regulatory approval. ResolveMass Laboratories Inc. offers comprehensive, regulatory-aligned peptide characterization services to help ANDA applicants demonstrate equivalence to the RLD with confidence.
Start your peptide analysis journey today with a lab that U.S. scientists trust.
1. What are the common impurities in peptides?

You can get in touch via our Contact Us page or email us directly to discuss your project.
🔎 How to Comply with U.S. Regulatory Requirements
Ensuring compliance with U.S. regulatory requirements for peptide characterization for generic projects submission in the United States is critical for pharmaceutical, biotech, and academic labs advancing peptides toward clinical or commercial applications. Whether you’re submitting to the FDA, filing an IND, or conducting GLP studies, non-compliance can result in costly delays or rejections.
Here’s a step-by-step guide to help you meet the essential regulatory standards for peptide characterization in the United States:
✅ Step 1: Understand Applicable Regulatory Frameworks
Peptide characterization service is governed by several U.S. regulatory standards, depending on the product lifecycle stage:
- FDA (Food and Drug Administration) – Especially for peptides classified as drug substances.
- USP (United States Pharmacopeia) – Offers testing monographs and quality benchmarks for peptide APIs.
- ICH Guidelines (Q6A, Q2(R1)) – Cover specifications, analytical validation, and impurity thresholds.
- GLP/GMP Requirements – For labs performing regulated studies or manufacturing-related testing.
💡 Pro Tip: Collaborate with an experienced lab that understands both research and regulatory contexts—like ResolveMass Laboratories Inc..
✅ Step 2: Select Validated Analytical Techniques
Regulatory bodies expect peptide analytical data to be determined using scientifically validated and sensitive techniques such as:
- HPLC (High-Performance Liquid Chromatography)
- UPLC (Ultra-Performance LC)
- LC-MS (Liquid Chromatography-Mass Spectrometry)
These methods should be validated for specificity, accuracy, precision, linearity, and robustness.
Refer to ICH Q2(R1) for a full validation protocol checklist.
📘 Learn more about our advanced peptide sequencing services that support impurity detection and regulatory documentation.
✅ Step 3: Document Impurities According to FDA Guidelines
According to FDA and ICH Q3A/B standards:
- Impurities >0.1% must be identified and reported.
- For peptides under IND/ANDA filings, all process-related impurities must be characterized.
- Degradation products must be distinguished from synthetic impurities.
You must also provide evidence of:
- Structural identity
- Toxicological relevance
- Source of impurity (synthesis vs degradation)
✅ Step 4: Implement GLP-Compliant Testing Procedures
GLP (Good Laboratory Practice) ensures data integrity and traceability. To comply:
- Use SOPs (Standard Operating Procedures) for each analytical method.
- Ensure all instruments are calibrated and qualified.
- Maintain audit-ready documentation for sample handling, analysis, and reporting.
ResolveMass Laboratories operates under GLP-compliant protocols, ensuring your characterization data is submission-ready.
👉 Get a quote for regulatory-compliant peptide testing.
✅ Step 5: Include Peptide Characterization data in CMC Documentation
If you’re filing for an Investigational New Drug (IND) or New Drug Application (NDA), peptide characterization must be included in your CMC (Chemistry, Manufacturing, and Controls) section.
The FDA expects:
- Clear description of testing methodology
- Validation data
- Batch-to-batch consistency analysis
- Impurity profiles over time (stability data)
Working with a lab experienced in regulatory submissions like ResolveMass can save you valuable time and ensure CMC data meets FDA scrutiny.
✅ Step 6: Stay Current with Regulatory Updates
U.S. and international regulatory bodies continually update expectations for biologics, peptides, and APIs. Subscribe to:
- FDA’s Drug Information Updates
- USP Monograph Revisions
- ICH Newsletters
Or, rely on testing partners like ResolveMass Laboratories Inc., which continuously aligns its protocols with the latest regulatory standards.